
����Ŀ��NH4Al(SO4)2��ʳƷ�ӹ�����Ϊ��ݵ�ʳƷ���Ӽ������ڱ���ʳƷ�У�NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺����ش��������⣺
��1��NH4Al(SO4)2������ˮ������������_____________________________(�ñ�Ҫ�Ļ�ѧ������������˵��)��
��2����ͬ�����£�0.1 mol��L��1NH4Al(SO4)2��c(![]() )________0.1 mol��L��1NH4HSO4��c(
)________0.1 mol��L��1NH4HSO4��c(![]() )��(����������������������������)
)��(����������������������������)
��3������ʽ��ʾ0.1 mol��L��1NH4Al(SO4)2��Һ�и�����Ũ�ȹ�ϵ____________��
��4����ͼ��0.1 mol��L��1�������Һ��pH���¶ȱ仯��ͼ��
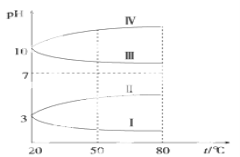
���з���0.1 mol��L��1NH4Al(SO4)2��pH���¶ȱ仯��������________(��д��ĸ)������pH���¶ȱ仯��ԭ����_____________________________��
��5������ʱ����100 mL 0.1 mol��L��1HCl��Һ�еμ�0.1 mol��L��1��ˮ���õ���ҺpH�백ˮ����Ĺ�ϵ������ͼ��ʾ��

���Է���ͼ��a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������____________��
����b�㣬��Һ�и�����Ũ���ɴ�С������˳����__________________;
��д��a������Һ��������ʽ�ľ�ȷ��������ܽ��Ƽ��㣩��
c��Cl-��- c��NH4+��=____________��c��H+��- c��NH3��H2O��=____________��
���𰸡�A13+ˮ�����ɵ�A1(OH)3������������ԣ���A13++3H2O= A1(OH)3(����)+3H+��A1(OH)3����������������ʹ������Ӷ�����ˮ С�� c(NH4+)+3c(Al3+)+c(H+)=2c(SO42-)+c(OH-) 1 NHAl(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����pH��С a c(Cl-)= c(NH4+)��c(OH-)=c(H+) 10-6-10-8�� 10-8
��������
(1)Al3+ˮ�����ɵ�Al(OH)3���壬���������ԣ�
(2)NH4Al(SO4)2��Al3+ˮ�����������NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣻
(3)��Һ�д��ڵ���غ������
(4)NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶���ˮ��̶�����
(5)��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a������Ͱ�ˮǡ����ȫ��Ӧ����Һ��ֻ��NH4Cl��b��c��d������Һ������NH3H2O���ݴ˷����жϣ���b����Һ�к���NH4Cl��NH3H2O�������ԣ�c(OH-)=c(H+)����ϵ���غ������𣻢�a��Ϊ�Ȼ����Һ�����ݵ���غ�������غ���㡣
(1)Al3+ˮ�����ɵ�Al(OH)3���壬�������������������������ԣ��ܹ�����ˮ���������ʴﵽ��ˮ��Ŀ�ģ�ˮ������ӷ���ʽΪ��Al3++3H2O=Al(OH)3����+3H+���ʴ�Ϊ��Al3+ˮ�����ɵ�Al(OH)3���壬���������ԣ���Al3++3H2O=Al(OH)3����+3H+��Al(OH)3������������ʹ������Ӷ�����ˮ��
(2)NH4Al(SO4)2��NH4HSO4�е�NH4+������ˮ�⣬����NH4Al(SO4)2��Al3+ˮ������Ի�����NH4+ˮ�⣬HSO4-�����H+ͬ������NH4+ˮ�⣬��ΪHSO4-�������ɵ�H+Ũ�ȱ�Al3+ˮ�����ɵ�H+Ũ�ȴ�����NH4HSO4��NH4+ˮ��̶ȱ�NH4Al(SO4)2�е�С���ʴ�Ϊ��С�ڣ�
(3)���ݵ���غ㣬��c(NH4+)+3c(Al3+)+c(H+)=2c(SO42-)+c(OH-)���ʴ�Ϊ��c(NH4+)+3c(Al3+)+c(H+)=2c(SO42-)+c(OH-)��
(4)NH4Al(SO4)2ˮ�⣬��Һ�����ԣ�pH��7�������¶ȣ�ˮ��̶�����pH��С�����ϵ�����Ϊ�ʴ�Ϊ����NH4Al(SO4)2ˮ�⣬��Һ�����ԣ������¶ȣ���ˮ��̶�����pH��С��
(5)��a��b��c��d�ĸ��㣬���ݷ�Ӧ���Ĺ�ϵ��a������Ͱ�ˮǡ����ȫ��Ӧ����Һ��ֻ��NH4Cl��b��c��d������Һ������NH3H2O��NH4Clˮ��ٽ�ˮ�ĵ��룬��NH3H2O����ˮ�ĵ��룬���ˮ�ĵ���̶�������a�㣬�ʴ�Ϊ��a��
��b����Һ�к���NH4Cl��NH3H2O�������ԣ�c(OH-)=c(H+)�����ݵ���غ㣬c(NH4+)= c(Cl-)����b��ʱc(Cl-)= c(NH4+)��c(OH-)=c(H+)���ʴ�Ϊ��c(Cl-)= c(NH4+)��c(OH-)=c(H+)��
��a��Ϊ�Ȼ����Һ�����ݵ���غ㣬c(Cl-)- c(NH4+)= c(H+)-c(OH-)=10-6-![]() =10-6-10-8�����������غ�c(H+)=c(NH3��H2O)+ c(OH-)�����c(H+)-c(NH3��H2O)= c(OH-)=10-8���ʴ�Ϊ��10-6-10-8��10-8��
=10-6-10-8�����������غ�c(H+)=c(NH3��H2O)+ c(OH-)�����c(H+)-c(NH3��H2O)= c(OH-)=10-8���ʴ�Ϊ��10-6-10-8��10-8��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����һ���������ォ��ˮ�е�����(K2NCONH2)�Ļ�ѧ��ֱ��ת��Ϊ���ܣ������ɶԻ��������ʵ�װ�D��ͬʱ���ô�װ�õĵ��������϶�ͭ������˵������ȷ����

A. H+�����ӽ���Ĥ���������ƶ�
B. ͭ�缫Ӧ��X������
C. M�缫��Ӧʽ��H2NCONH2+H2O-6e-== CO2��+N2��+6H+
D. ��N�缫����0.25 mol����ʱ�����缫����16 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������G��һ�ֿ���������������ҩ�ʵ�����ɷ�����A�Ʊ�G�ĺϳ�·����ͼ��
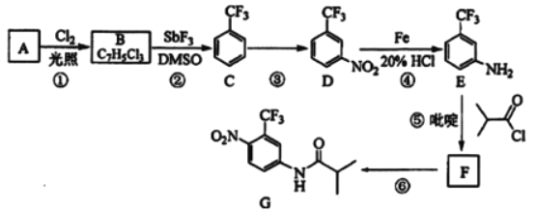
�ش��������⣺
��1��A�Ľṹ��ʽΪ__��C�Ļ�ѧ������__��
��2���۵ķ�Ӧ�Լ��ͷ�Ӧ�����ֱ���__���÷�Ӧ��������__��
��3���ݵķ�Ӧ����ʽΪ__�������һ���л����������__��
��4��G�ķ���ʽΪ__��
��5��H��G��ͬ���칹�壬�䱽���ϵ�ȡ������G����ͬ��λ�ò�ͬ����H���ܵĽṹ��__�֡�
��6��4��������������(![]() )����Ҫ�ľ�ϸ�����м��壬д���ɱ�����(
)����Ҫ�ľ�ϸ�����м��壬д���ɱ�����(![]() )�Ʊ�4���������������ĺϳ�·��__(�����Լ���ѡ)��
)�Ʊ�4���������������ĺϳ�·��__(�����Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ũ��Ϊ0.1mol��L-1HF��Һ��ˮ����ϡ�ͣ����и���ʼ�ձ����������
A.c��H+��B.Ka��HF��C.c(F-��/c(H+��D.c(F-��/c(HF��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ĸ�ͼ������ӳ����������Ӧ��Ӧ���ϵ���(a��b��c��d������0)�� ��
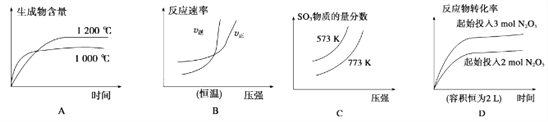
A. 4NH3(g)��5O2(g)![]() 4NO(g)��6H2O(g)��H��-akJ��mol-1
4NO(g)��6H2O(g)��H��-akJ��mol-1
B. N2(g)��3H2(g)![]() 2NH3(g)��H��-bkJ��mol-1
2NH3(g)��H��-bkJ��mol-1
C. 2SO3(g)![]() 2SO2(g)��O2(g)��H��+ckJ��mol-1
2SO2(g)��O2(g)��H��+ckJ��mol-1
D. 2N2O5(g)![]() 4NO2(g)��O2(g)��H��+dkJ��mol-1
4NO2(g)��O2(g)��H��+dkJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����( )
A. ��֪25 ��ʱNH4CN��Һ�Լ���,��25 ��ʱ�ĵ��볣��K(NH3��H2O)>K(HCN)
B. ����ʱ, ��ͬ�������ͬpH������ʹ�����Һ����ˮ�������c(H+):����С�ڴ�����Һ
C. ��֪Ksp(AgCl)=1.56��10-10,Ksp(Ag2CrO4)=9.0��10-12,����Cl-��CrO42-��Ũ�Ⱦ�Ϊ0.010 mol��L-1��Һ����μ���0.010 mol��L-1��AgNO3��Һʱ, CrO42-�Ȳ�������
D. ������pH=7��CH3COOH��NaOH�����Һ��,c(Na+)>c(CH3COO-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��BaCl2��xH2O����;�㷺�Ļ���������Ʒ���ҹ�Ŀǰ��Ҫ�����������(������Mg2����Fe3����)��Ӧ����BaCl2��xH2O������������ͼ��ʾ����ش�
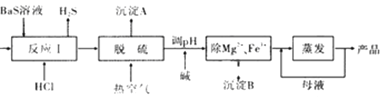
��֪������ʱKsp[Mg(OH)2]=1.8��10��11��Ksp[Fe(OH)3]=4.0��10��38
��1����ӦI�����ɵ�H2S��������ˮ���գ�һ����������������Һ��ͨ�˿������ֿɵõ�������ʹ����Һ������������Ӧ�Ļ�ѧ����ʽΪ_________��
��2�������Ȼ�����Һ�к�����(H2S��HS����)Ӱ���Ʒ�������ɹ���Ԥ�Ⱥ�Ŀ���������Ԥ�ȿ�����Ŀ����_________������A����Ҫ�ɷ���_________��
��3���ȿ�������ʱ���в���HS��ת��ΪS2O32����ʹ��Ʒ�Բ��ܴﵽ����Ҫ�������ữ�����ữ����ʱ�����ӷ���ʽΪ_________��
��4������ʱ��ΪʹMg2����Fe3����ȫ����(����Һ������Ũ��С��1��l0��5mol![]() ʱ��Ϊ��������ȫ����)��Ӧ����Һ��pH����_________(ֻ����ʽ)���ϡ�
ʱ��Ϊ��������ȫ����)��Ӧ����Һ��pH����_________(ֻ����ʽ)���ϡ�
��5��ʵ���Ҳⶨ��Ʒ��x�IJ������£�
��ȷ��ȡ12.23 g BaCl2��xH2O��Ʒ������l00 mLϡ��������ܽ⣻
���߽��裬����μ���0.lmol![]() H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2��3�Σ�
H2SO4��Һ����BaSO4��ȫ���������ˣ�������ϴ�ӳ���2��3�Σ�
����������ָ�������������Ϊ11.65 g������BaSO4�����Ƿ�ϴ�Ӹɾ��ķ�����_______��������x����ֵΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��B��C��D���ֳ����Ľ������ʣ�A�ڿ�����ȼ�����ɵ���ɫ���壻BΪ��ɫ���壬��ʴʱ��Ϊ��ɫ��C�ڿ����м����ۻ��������䣻D��������ȼ�գ��������䡣
����������Ϣ�ش��������⣺
(1)д����Ӧ��ѧʽ��A______��B______��C______��D______��
(2)A����������������Ӧʱ����__________��д��ѧʽ����ͬ����D�ڿ�������ʴ����__________��
(3)д�����л�ѧ����ʽ��
��A�ڿ�����ȼ��______________________��
��B�������ڼ��������·�Ӧ______________________��
��C��������ȼ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�1��4���ڣ�����Aԭ�Ӻ���һ�����ӣ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������η��ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����ش��������⣺
��1��������Ԫ���е縺��������____(��Ԫ�ط��ţ���ͬ)����һ��������С����____��
��2��C���ڵ�����Ԫ����̬�⻯���У��е���͵���____(�ѧʽ)��
��3��BԪ�ؿ��γɶ��ֵ��ʣ����С�ֻ��һ��ԭ�Ӻ����ʣ�������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ��ò��Ͼ���ṹ��ͼ��ʾ����ԭ�ӵ��ӻ�����Ϊ______��
��4��D�Ĵ����ξ���ֲ��ṹ��ͼ���þ����к��еĻ�ѧ����____(��ѡ�����)��

�ټ��Լ��� �ڷǼ��Լ� ����λ������ �ܽ�����
��5��ijѧ���������й�DԪ�ص�ʵ����������ͼ��
D����![]() ��ɫ����
��ɫ����![]() ��ɫ��Һ
��ɫ��Һ![]() ��ɫ����
��ɫ����![]() ��ɫ��Һ
��ɫ��Һ![]() ��ɫ����
��ɫ����
����д�ڢݲ���Ӧ�����ӷ���ʽ��_____________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com