
����Ŀ��ijУ����С��Ϊ�ⶨij̼���ƺ�̼�����ƻ������̼���Ƶ������������ס�������ͬѧ�ֱ�������������ʵ�顣
��������ͬѧ��������������ͼ��ʾ��ʵ�����̽���ʵ�飺
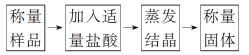
��1��ʵ��ʱ�������ᾧ�����У���������̨(����Ȧ)���ƾ����⣬��Ҫ�õ���������________��
��2����ͬѧ��Ϊ�������������ᡱ���ÿ��ƣ�Ӧ��Ϊ������������ᡱ�����ڲ����Ҳ�Ӱ��ⶨ��ȷ�ԣ��ù۵�________(���ȷ������ȷ��)����ԭ����___________________________��
��3����ʵ���в����Ʒ����Ϊ46.4g���������������Ϊ40.95g����̼���Ƶ���������Ϊ________(����3λ��Ч����)��
��4�������ᾧ���������й���ɽ������̼���Ƶ���������________(�ƫ��ƫС������Ӱ�족)��
����������ͬѧ����Ҫʵ���������£�
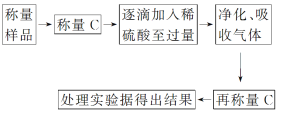
����ͼ��ʾװ�ý���ʵ�飺
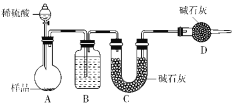
��5��װ��B�е��Լ�Ϊ_______��װ��C��װ��ʯ�������վ���������壬װ��D��������________________________��
��6���е�ͬѧ��ΪΪ�˼�Сʵ�����ڷ�Ӧǰ��ͨ��N2����Ӧ��ͨ��N2��Ŀ����________________________________________________________________________��
�����������������
��7����һ������Ʒ������ϡ���ᷴӦ������ͼװ�ò�������CO2����������B����Һ��ò���________(�����)ʹ��������С��

a������̼������Һ b������̼��������Һ
c����������������Һ d����������ͭ��Һ
���𰸡����������� ��ȷ �����ӷ��������������ڼ��ȹ����лӷ�����Ӱ���� 45.7% ƫС Ũ���� ���տ����е�ˮ�����Ͷ�����̼����ȷ��U�ι���������������ȷ�� ʹA��Bװ���в�����CO2ȫ����Cװ���еļ�ʯ�����գ���Сʵ����� b
��������
������̼����������̼����������֮��Ϊ��Ʒ����������Ʒ��Һ�м������ᣬ̼���ƺ����ᷴӦ�����Ȼ�����Һ��̼�����������ᷴӦ�����Ȼ�����Һ�������ᾧ����NaCl���壬����NaCl��������������Ȼ��Ƶ���������Ʒ�������ɼ���̼����������̼�������������������̼���Ƶ�����������
������̼����������̼����������֮��Ϊ��Ʒ����������Ʒ�����ᷴӦ����������̼���壬�ü�ʯ�ҽ�������̼���գ���ʯ�����ӵ�������Ϊ������̼�������������Ʒ��������������̼�����ɼ���̼���Ƶ��������������̼���Ƶ�����������
���������Ʒ����������������̼���������̼���Ƶ��������Ӷ�����̼���Ƶ�����������
(1)�����ᾧ�����У���Ҫ�����ƾ��ơ��������ȣ��ʴ�Ϊ��������������
(2)�ɷ�����֪���÷���ԭ��������Ʒ�������ͷ�Ӧ�������Ȼ��Ƶ���������̼���Ƶ��������Ӷ�����̼���Ƶ�������������ô��ʵ��Ĺؼ��ǽ�̼���ơ�̼��������ȫת��Ϊ�Ȼ��ƣ���Ϊ���������ᡱ����ʹ̼���ơ�̼��������ȫת��Ϊ�Ȼ��ƣ����ڲ������������ӷ��������������ڼ��ȹ����лӷ�����������Ӱ���������Ըù۵���ȷ���ʴ�Ϊ����ȷ�������ӷ��������������ڼ��ȹ����лӷ�����Ӱ������
(3)��ʵ���в����Ʒ����Ϊ46.4g����������Ϊ40.95g����̼�������ʵ���Ϊx��̼���������ʵ���Ϊy����![]() ���ɽ��Na+�غ��У�
���ɽ��Na+�غ��У�![]() ��������ʽ��ã�
��������ʽ��ã�![]() ��
��![]() ��̼���Ƶ���������Ϊ
��̼���Ƶ���������Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() �����ᾧ���������й���ɽ����Ȼ����������٣���������̼����������С����̼��������������С���ʴ�Ϊ��ƫС��
�����ᾧ���������й���ɽ����Ȼ����������٣���������̼����������С����̼��������������С���ʴ�Ϊ��ƫС��
![]() A�е�ˮ�����ܱ�C�м�ʯ�����գ�����ʵ��������B�������Ƿ�ֹA�е�ˮ��������C�У���B�п�ʢ��Ũ���ᡣ�����е�ˮ�����Ͷ�����̼�ᱻ��ʯ�����գ���D�����������տ����е�ˮ�����Ͷ�����̼����ȷ��U�ι���������������ȷ�ԣ��ʴ�Ϊ��Ũ���ᡢ���տ����е�ˮ�����Ͷ�����̼����ȷ��U�ι���������������ȷ�ԣ�
A�е�ˮ�����ܱ�C�м�ʯ�����գ�����ʵ��������B�������Ƿ�ֹA�е�ˮ��������C�У���B�п�ʢ��Ũ���ᡣ�����е�ˮ�����Ͷ�����̼�ᱻ��ʯ�����գ���D�����������տ����е�ˮ�����Ͷ�����̼����ȷ��U�ι���������������ȷ�ԣ��ʴ�Ϊ��Ũ���ᡢ���տ����е�ˮ�����Ͷ�����̼����ȷ��U�ι���������������ȷ�ԣ�![]() �÷����ؼ���Ҫ��ò�����
�÷����ؼ���Ҫ��ò�����![]() ��������ʵ��ǰ�����ں��п����������к��ж�����̼��ˮ��������Ӱ��C����������Ӧ��װ���������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ������Է�Ӧǰ��Ҫͨ��
��������ʵ��ǰ�����ں��п����������к��ж�����̼��ˮ��������Ӱ��C����������Ӧ��װ���������ں��ж�����̼�����ܱ�C�м�ʯ����ȫ���գ����²ⶨ����нϴ������Է�Ӧǰ��Ҫͨ��![]() ����Ӧ��ͨ��
����Ӧ��ͨ��![]() ��Ŀ���ǣ�ʹA��Bװ���в�����CO2ȫ����Cװ���еļ�ʯ�����գ���Сʵ�����ʴ�Ϊ��ʹA��Bװ���в�����CO2ȫ����Cװ���еļ�ʯ�����գ���Сʵ����
��Ŀ���ǣ�ʹA��Bװ���в�����CO2ȫ����Cװ���еļ�ʯ�����գ���Сʵ�����ʴ�Ϊ��ʹA��Bװ���в�����CO2ȫ����Cװ���еļ�ʯ�����գ���Сʵ����
(7)���òⶨ��Ӧ���ɵĶ�����̼��������ķ����ⶨ̼���Ƶ������������ؼ�����ȷ������Ӧ���ɵĶ�����̼���������ô������̼��B�е��ܽ��ԽС��Խ����ӦԽ�ã������������DZ���̼��������Һ���ʴ�Ϊ��b��


 â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���л�����C��H��O����Ԫ����ɣ���������ģ����ͼ��ʾ�������й�������ȷ���ǣ� ��
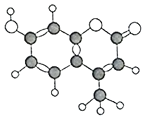
�ٷ���ʽΪC10H9O3
�ڸ÷�����10��̼ԭ�Ӷ�������ͬһƽ����
��1mol�������������5mol H2�ӳ�
�ܸ�������ʹ����KMnO4��Һ��ɫ
��1mol������������뺬3mol NaOH����Һ��Ӧ
��1mol������������Ũ��ˮ��Ӧ���������3mol Br2
�߸�������FeCl3��Һ����ɫ
��������ܺ�Na2CO3��Һ��NaHCO3��Һ��Ӧ
A.�٢ڢܢ�B.�ڢܢݢ�C.�ڢܢߢ�D.�ڢۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
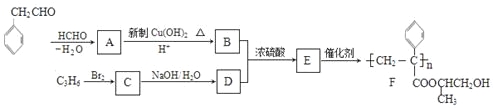
����Ŀ������Ϊ�ϳɾۺ���F��·��ͼ��
![]()
�����������Ϣ���ش��������⣺
��1��A�к��й����ŵ�������___________________��C��ϵͳ������___________��
��2��B+D��E�ķ�Ӧ������_______��
��3��C����D�ķ�Ӧ��ѧ����ʽΪ__________�����C��NaOH�Ĵ���Һ��Ӧ����Ӧ����Ϊ__________��
��4��G���ʵ���Է���������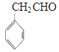 ����Է���������2�����������������G��ͬ���칹����____�֡�
����Է���������2�����������������G��ͬ���칹����____�֡�
�ٷ����к��б������ұ�����������ȡ����
�����Ȼ�����Һ����ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A ��ʯ���ѽ�������Ҫ�ɷ�֮һ���� A Ϊԭ���Ʊ�ҩ���м��� X �ĺϳ�·�����£�

��֪�� i 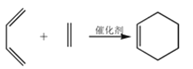 ii
ii ![]()
�ش��������⣺
(1)D �к�̼ԭ�ӵĹ����ŵĽṹ��ʽΪ_____��
(2)�л��� I �Ľṹ��ʽΪ_____����Ӧ�ܵķ�Ӧ������________��
(3)��Ӧ�Ļ�ѧ����ʽΪ_____��
(4)������֪����д�� CH2=CH��CH=CH2 ���л��� G �Ļ�ѧ��Ӧ����ʽ_____�� �ò������Ӧ�ۺ�õ����л�����˴Ź�����������_____��塣
(5)�������������� I ��ͬ���칹����_____�֡�
i �����ŵ������� I ��ͬ�� ii ������Ԫ���ṹ�� iii ��Ԫ����ֻ�� 1 ��ȡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵ���.ʳ.ס.�о��뻯ѧ�������е���ϵ��
(1)��װ��������ࡣ���������в�������Ȼ��ά����________(�����)��
a�����顡��b��˿��ë����c�����ںͽ���
(2)Ӫ���������������彡����ά������������Ҫ��Ӫ�����ʡ���ͼΪijƷ��ά����C����Ƭ˵����IJ������ݡ�������Ƭ�����ӵ���ɫ����________����ζ����________��
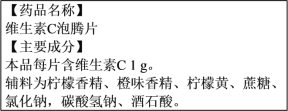
(3)�����ͷɻ�����Ҫ�Ľ�ͨ���ߣ���������̥����Ҫ������________(�����)��
a������ b���� c�������� d���ѺϽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ˮ��������ͼ��ʾ���ش��������⣮
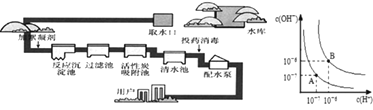
![]() �dz��õ�������������ˮ���������� ______ ����
�dz��õ�������������ˮ���������� ______ ����![]() �ѧʽ
�ѧʽ![]() ��
��
![]() ��Ȼˮ�к��н϶�ĸơ�þ���ӣ���ΪӲˮ�������г��� ______ ����������ˮ��Ӳ�ȣ�
��Ȼˮ�к��н϶�ĸơ�þ���ӣ���ΪӲˮ�������г��� ______ ����������ˮ��Ӳ�ȣ�
![]() ���˳�������ʵ���ҹ���ԭ������ʵ���ҹ��˲������õ��IJ��������� ______ ��
���˳�������ʵ���ҹ���ԭ������ʵ���ҹ��˲������õ��IJ��������� ______ ��
![]() ��Ͷҩ�������е���ҩ��ָƯ�ۣ���д����ȡƯ�۵Ļ�ѧ����ʽ ______ ��
��Ͷҩ�������е���ҩ��ָƯ�ۣ���д����ȡƯ�۵Ļ�ѧ����ʽ ______ ��
![]() ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ
ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ![]() ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ
ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ![]() ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�
ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�
![]() ʱ
ʱ![]() ��NaOH��Һ�У���ˮ�������
��NaOH��Һ�У���ˮ�������![]() ______
______ ![]() ��
��![]() ______
______ ![]() ����
����![]() ����
����![]() ������
������![]() ��
��![]() ��
��
![]() ʱ����100mlˮ���ձ�������һ�����ı����ᣬ����������ȷ���� ______
ʱ����100mlˮ���ձ�������һ�����ı����ᣬ����������ȷ���� ______ ![]() ����ĸ
����ĸ![]() ��
��
A.��Һ��һֱ���ڣ�![]()
B.��������У�ˮ�ĵ���̶���С������ĵ���̶�������
C.��������У���Һ����������Ũ��������
D.�����������������ձ��¶ȣ���ҺpHֵ����
E.����֪Ũ�ȵ�NaOH��Һ�ζ��ձ��еĴ�����Һ���ⶨ��Ũ�ȣ����ѡ�ü�����ָʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ɶ�����Ԫ����ɵij����Ĵ��������֮��ת����ϵ��ͼ��ʾ(������ˮ��Һ�з�Ӧ)
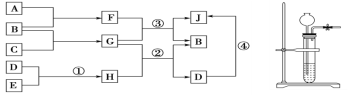
a��A��B��H�ڳ����¾�����̬��FΪҺ̬��EΪ���ᣬDΪ����ˮ�İ�ɫ���壬A��B��CΪ���ʣ�A��B��ȼ�ղ�������ɫ���档
b��ʵ������ȡA��H�ķ���װ����ͼ��ʾ��
c���������������£���GͶ���̷���Һ�У��к��ɫ�������ɣ��������������ݡ��ش��������⣺
��1��������G���������������Ӹ���֮��Ϊ____________��J��������ѧ��������____________________________________________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
��_____________________________________________________��
��_____________________________________________________��
��3����Ӧ�ٵ����ӷ���ʽ��____________________________________________
��4��������G����Ҫ��;����;Ϊ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������س��õ�NaNO2����ۺ�ʳ�����ƣ�������ζ������ʹ����ʳ�ж�����֪![]() �ܷ������·�Ӧ
�ܷ������·�Ӧ
_______NaNO2+_____HI����_________NO��+_________I2+_______NaI+____H2O
(1)��ƽ���淽��ʽ��
(2)����1 mol������������ԭ���������Ļ�ԭ���� ________mol��
(3)����������Ӧ��������ֽ�������г��������ʽ���ʵ�飬�Լ���NaNO2��NaCl����ѡ�õ������У���ˮ �ڵ⻯�ص�����ֽ �۵��� �ܰ� ��ʳ�ף�����ʵ�飬����ѡ����ʵ���_____(����ĸ)��
A.�٢ڢ� B.�ۢ� C. �٢ڢۢ� D.�٢ڢ�
(4)ij����Һ�У�����2%��5%��NaNO2��ֱ���ŷŻ������Ⱦ������NH4Cl����ʹNaNO2ת��Ϊ�����������Ⱦ��N2����Ӧ�Ļ�ѧ����ʽΪ_______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����˾ƥ�֣�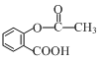 ����Ӧ�ù㷺�Ľ�����ʹҩ��ͨ���Ҷ�������˾ƥ�������ڸ߾���E�ϣ����Ƶû��ͳ�Ч��˾ƥ�֣���ϳ�·����ͼ�����ַ�Ӧ��������ȥ����
����Ӧ�ù㷺�Ľ�����ʹҩ��ͨ���Ҷ�������˾ƥ�������ڸ߾���E�ϣ����Ƶû��ͳ�Ч��˾ƥ�֣���ϳ�·����ͼ�����ַ�Ӧ��������ȥ����
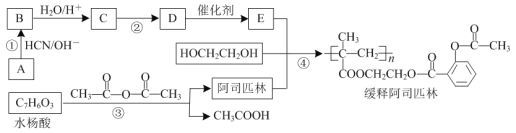
��֪��CH3CHO![]()
�ش��������⣺
��1����Ӧ�ٵķ�Ӧ������___��
��2����Ӧ�ڵĻ�ѧ����ʽΪ___��
��3��д��ͬʱ�������������İ�˾ƥ�ֵ�һ��ͬ���칹��Ľṹ��ʽ��___��
��.���ڷ����廯�������NaHCO3��Һ��Ӧ��
��.�ܷ���ˮ�ⷴӦ������������ˮ�������л����о��������ֲ�ͬ��ѧ�������⡣
��4��д����![]() Ϊԭ���Ʊ�ҩ���м���Y(
Ϊԭ���Ʊ�ҩ���м���Y(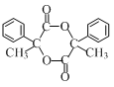 )�ĺϳ�·������ͼ___(���Լ����ã��ϳ�·������ͼʾ�����������)��
)�ĺϳ�·������ͼ___(���Լ����ã��ϳ�·������ͼʾ�����������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com