

 +H2O
+H2O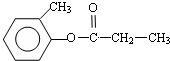 ��
��
 ��
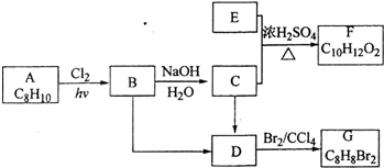
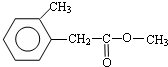
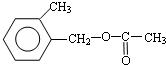
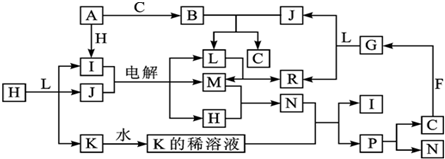
�� ���� A����ʽΪC8H10��Ϊһȡ���������������Ͷ�Ϊ$\frac{2��8+2-10}{2}$=4��Ϊ����ͬϵ���AΪ ��A�������ڹ��������·���ȡ����Ӧ����B����B�к���һ��������BΪ
��A�������ڹ��������·���ȡ����Ӧ����B����B�к���һ��������BΪ ��B����ˮ�ⷴӦ
��B����ˮ�ⷴӦ ������CΪ
������CΪ ��
��
E������������������ܶ�Ϊ30����Mr��E��=30��2=60��6.0gE�����ʵ�����0.1mol����ȫȼ�պ�����CO2�� H2O�����ʵ����ֱ�Ϊ$\frac{8.8g}{44g/mol}$=0.2mol��$\frac{3.6g}{18g/mol}$=0.2mol��������N��C��=$\frac{0.2mol}{0.1mol}$=2��N��H��=$\frac{0.2mol��2}{0.1mol}$=4����N��O��=$\frac{60-12��2-8}{16}$=2����E�ķ���ʽ��C2H4O2��
C��E����������Ӧ����F�����F�ķ���ʽ��֪��Ӧ�Ƿ���������Ӧ����EΪCH3COOH��FΪ ��B��Cת�����õ�D��D���巢���ӳɷ�Ӧ����G����B��C��������ȥ��Ӧ����D����DΪ��C6H5CH=CH2����GΪ
��B��Cת�����õ�D��D���巢���ӳɷ�Ӧ����G����B��C��������ȥ��Ӧ����D����DΪ��C6H5CH=CH2����GΪ ���ݴ˽��
���ݴ˽��
��1�����ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ�E������������������ܶ�Ϊ30��Ħ��������60���������ɶ�����̼��ˮ������ȷ���л���ΪC2H4O2��
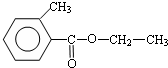
��2���ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C8H10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC6H5CHClCH3��C��E����������Ӧ����F��EΪCH3COOH�����F�ķ���ʽ��֪��FΪC6H5CHOHCH3���ݴ���д��ѧ����ʽ��
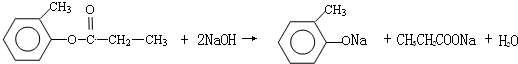
��3����ΪD������Br2�����ӳɷ�Ӧ������D�DZ���ϩ���ṹ��ʽΪC6H5CH=CH2��B��DΪ±������NaOH����Һ�����·�����ȥ��Ӧ��E+C��FΪCH3COOH��C6H5CHOHCH3������Ӧ���ݴ���д��ѧ����ʽ��
��4������ʽΪC10H12O2���л����F������ͬ�Ĺ����ţ��������Ľṹ��ֻ��������λȡ����������һ��Ϊ�����ݴ��ж�ͬ���칹���������д��ѧ����ʽ��
��� �⣺��1�����ݰ����ӵ����ɵ���������ܶ�֮�ȵ���Ħ������֮�ȣ�E������������������ܶ�Ϊ30����Mr��E��=30��2=60��6.0gE�����ʵ�����0.1mol����ȫȼ�պ�����CO2��H2O�����ʵ����ֱ�Ϊ$\frac{8.8g}{44g/mol}$=0.2mol��$\frac{3.6g}{18g/mol}$=0.2mol��������N��C��=$\frac{0.2mol}{0.1mol}$=2��N��H��=$\frac{0.2mol��2}{0.1mol}$=4����N��O��=$\frac{60-12��2-8}{16}$=2����E�ķ���ʽ��C2H4O2���ʴ�Ϊ��C2H4O2��
��2���ɿ�ͼ��֪E��C��������F����ԭ���غ�֪C�ķ���ʽ��C8H10O����ΪAΪһȡ����������B�к���һ����������B�Ľṹ��ʽΪC6H5CHClCH3��B����ˮ�ⷴӦ ������CΪ
������CΪ ��E�ķ���ʽ��C2H4O2��C��E������Ӧ����F�����F�ķ���ʽ��֪��EΪCH3COOH��FΪC6H5CHOHCH3��������B����C�Ļ�ѧ����ʽΪC6H5CHClCH3+H2O$��_{��}^{NaOH}$C6H5CHOHCH3+HCl��
��E�ķ���ʽ��C2H4O2��C��E������Ӧ����F�����F�ķ���ʽ��֪��EΪCH3COOH��FΪC6H5CHOHCH3��������B����C�Ļ�ѧ����ʽΪC6H5CHClCH3+H2O$��_{��}^{NaOH}$C6H5CHOHCH3+HCl��
�ʴ�Ϊ��C6H5CHClCH3+H2O$��_{��}^{NaOH}$C6H5CHOHCH3+HCl��
��3��B�Ľṹ��ʽΪC6H5CHClCH3��B��DΪ±������NaOH����Һ�����·�����ȥ��Ӧ����ӦΪ��
C6H5CHClCH3+NaOH$��_{��}^{��}$C6H5CH=CH2+NaCl+H2O��EΪCH3COOH��CΪ ��FΪC6H5CHOHCH3��
��FΪC6H5CHOHCH3��
E+C��FΪCH3COOH��C6H5CHOHCH3������Ӧ����ӦΪ��C6H5CHOHCH3+CH3COOH
 +H2O��
+H2O��
�ʴ�Ϊ��C6H5CHClCH3+NaOH$��_{��}^{��}$C6H5CH=CH2+NaCl+H2O��C6H5CHOHCH3+CH3COOH
 +H2O��
+H2O��
��4������ʽΪC10H12O2���л����F������ͬ�Ĺ����ţ��������Ľṹ��ֻ��������λȡ����������һ��Ϊ��������������ͬ���칹��Ϊ

 ��5�֣�����������������Һ��Ӧʱ����2mol�������ƣ��䷴Ӧ����ʽΪ��
��5�֣�����������������Һ��Ӧʱ����2mol�������ƣ��䷴Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ��



 ��
�� ��
��
���� ������Ҫ�����л���ṹʽ��ȷ�����л���������ƶϡ�ͬ���칹�����д���жϡ��л���Ӧ����ʽ����д���״���Ϊͬ���칹����жϣ�ע����ȷ�ƶ��л���ĽṹΪ������Ĺؼ�����Ҫѧ���������չ����ŵ�������ת������Ŀ�ѶȽϴ�


 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������믣�XeF6�� | B�� | �����ᣨHClO�� | C�� | ��������BF3�� | D�� | ���� ��N2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
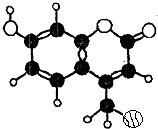 ij�ּ���Ⱦ�ϣ�Ӧ���ڿɵ�гȾ�ϼ�����������C��H��O����Ԫ����ɣ���һ�����ķ����������ͼ��ʾ�������йظ�������������ȷ���ǣ�������
ij�ּ���Ⱦ�ϣ�Ӧ���ڿɵ�гȾ�ϼ�����������C��H��O����Ԫ����ɣ���һ�����ķ����������ͼ��ʾ�������йظ�������������ȷ���ǣ�������| A�� | �٢ڢ� | B�� | �ۢܢ� | C�� | �ڢۢ� | D�� | �ڢۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | �÷�Ӧ�ų�251.2 kJ������ | B�� | �÷�Ӧ����251.2 kJ������ | ||
| C�� | �÷�Ӧ�ų�125.6 kJ������ | D�� | �÷�Ӧ����125.6 kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  2-�һ����� | B�� |  3-����-3-��ϩ | C�� |  2��4-���������� | D�� |  2��3-������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
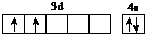
| a | ԭ�Ӻ�����ӷֱ�ռ3����ͬ�ܼ�����ÿ���ܼ����Ų��ĵ�������ͬ |
| b | ��̬ԭ�ӵ�p�����������s�����������1 |
| c | �����ڱ�����Ԫ���е縺����� |
| d | λ�����ڱ��е�4���� |
| e | ��̬ԭ��M��ȫ������N��ֻ��һ������ |
 ��
��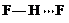 ��
��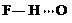 ��
��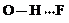 ��
��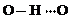 ����һ�֣�
����һ�֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com