
| A��һ������Mg2+��Al3+��Cl��������Na+��NH4+ |
| B��һ������Na+��Mg2+��Cl��������NH4+�����ܺ���Al3+ |
| C��c (Cl��) Ϊ 4.00 mol��L-1��c (Al3+) Ϊ1.00 mol��L-1 |
| D��c (Mg2+) Ϊ 1.00 mol��L-1��c(Na+ ) Ϊ 0.50 mol��L-1 |
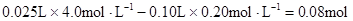 ��n(Mg2+)=
��n(Mg2+)=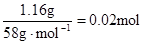 Mg2+ + 2OH��= Mg(OH)2������Mg2+��Ӧ��OH����
Mg2+ + 2OH��= Mg(OH)2������Mg2+��Ӧ��OH����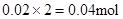 ������ԭ��Һ��һ������Al3+����Al3+��Ӧ��OH����
������ԭ��Һ��һ������Al3+����Al3+��Ӧ��OH����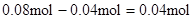 ������Һ�л���OH����������Ӧ Al3+ + 4OH��= AlO2�� + 2H2O ��n(Al3+ )=
������Һ�л���OH����������Ӧ Al3+ + 4OH��= AlO2�� + 2H2O ��n(Al3+ )= 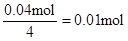 ����ʵ��ڽ������ļ������ݣ��� n(Cl��)=
����ʵ��ڽ������ļ������ݣ��� n(Cl��)= 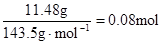 �� n(Cl��)��2n(Mg2+) + 3n(Al3+ ) ����ԭ��Һ�л����� Na+ ��2n(Mg2+) + 3n(Al3+ )+ n(Na+ ) =
�� n(Cl��)��2n(Mg2+) + 3n(Al3+ ) ����ԭ��Һ�л����� Na+ ��2n(Mg2+) + 3n(Al3+ )+ n(Na+ ) = 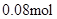 n(Na+ ) =
n(Na+ ) = 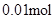
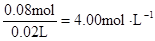 c (Al3+)=
c (Al3+)=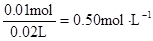
 c(Na+ )=
c(Na+ )= 


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Һ��pH=13 |
| B����������NaOH����Һ��c(NH4+)��С��Kw��С |
| C����AlCl3��Һ��Ӧ�����ӷ���ʽΪ Al3++3OH�D=Al(OH)3�� |
| D���μ�0.10 mol��L-1HNO3��Һ��pH=7����Һ������Ũ�ȹ�ϵΪ��c (NO3�D) = c(NH4+)> c(H+)=c(OH�D) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NH4+��Ba2+��NO3����CO32�� | B��K+��Mg2+��NO3����SO42�� |
| C��Fe2+��OH����SO42����MnO4�� | D��Na+��Fe3+��Cl����AlO2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2CO3��Һ��c(Na+)��c(CO32��)֮��Ϊ2:1 |
| B��pH��2��pH��1��������c(H+)֮��Ϊ1��10 |
| C��0.2 mol?L-1��0.1mol/L������c(H+)֮��Ϊ2:1 |
| D����ͬ�����0.1 mol?L-1�����0.1mol/L����ֱ�������þ����Ӧ���ų�H2������ȴ���1:1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ��ѧʽ | CH3COOH | H2CO3 | HClO | H2C4H4O6(��ʯ��) | H2SO3 |
| ����ƽ�ⳣ�� | 2.0��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 | K1=9.1��10-4 K2=4.3��10-5 | K1=1.3��10-2 K2=6.3��10-8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʹ�⻯�ص�����ֽ��������Һ��Na����NH4+��S2����Br�� |
| B����pH��ֽ���ɫ����Һ��Fe2+��S2O32-��SO42����Na+ |
| C��ˮ�������c(H��) ?c(OH��) =10��28����Һ��Na����S2����NO3����SO32�� |
| D����ʹKSCN�Ժ�ɫ����Һ��Na����NH4����AlO2-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��H+��I����NO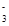 ��SiO32- ��SiO32- | B��NH4+��OH����Cl����HCO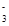 |
C��K+��SO42-��Cu2+��NO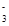 | D��Al3+��Mg2+��SO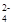 ��CO ��CO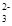 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��pH=1����Һ�У�NH4+��Fe2+��SO42����ClO�� |
| B��ͨ�����SO2��������Һ�У�K+��Na+��CO32����SO42�� |
| C��AlO2һ ��Ũ��Ϊ0.1 mol/L����Һ�У�K+��Na+��SiO32����SO42�� |
| D�������£���ˮ�������c(H+)=1��10��11 mol/L����Һ�У�Fe3+��Br����Cl����SCN�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʹ����-KI��ֽ����ɫ����Һ�У�K+ SO42- S2- SO32- |
| B������0��1mol��L-1 Fe2+����Һ��Na+Cl- ClO- SO42- |
| C��c��H+��/c��OH-��=1012����Һ�У�Al3+ NH4+ NO3- K+ |
| D������0��1mol��L-1 HCO3-����Һ��Na+ Fe3+ NO3- C6H5O- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com