
����Ŀ��I��ʵ�����г���MnO2����Ũ����ķ�����ȡ������ʵ��װ����ͼ��ʾ��
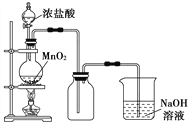
(1)Բ����ƿ�з�����Ӧ�Ļ�ѧ��Ӧ����ʽ��__________________��
(2)�������������������20 mL 12 mol��L��1�������ϼ��ȣ���������Ļӷ�������ַ�Ӧ�����ɵ���������_________������ڡ����ڡ�С�ڣ�0.06 mol������Ҫԭ����_____________________________��
(3)д��β�����������ӷ���ʽ��_______________________��
II����Na2CO3��10H2O���壬����0.2 mol��L��1��Na2CO3��Һ480 mL��
��1��Ӧ��ȡNa2CO3��10H2O�����������____________������ʱ��������ƿ�м�ˮ����1��2cmʱ������_________��ˮ���̶ȣ��Ӹ�ҡ�ȣ�
��2�����в�����������Һ��Ũ�ȿ��ܲ���Ӱ��
��Na2CO3��10H2O����ʧȥ�˲��ֽᾧˮ�����á���������ij���������������(ʹ������)����̼���ƾ��岻�������л����Ȼ��ơ�������ƿδ������ʹ�á� ��������������ҺŨ��ƫ�ߵ���______________(�����)��
���𰸡�MnO2+4HCl�� Ũ��![]() MnCl2+Cl2��+2H2OС���淴Ӧ�Ľ��У�����Ũ�ȱ�С��ϡ����Ͷ������̲���ӦCl2+2OH�C=Cl�C+ClO�C+H2O28.6g��ͷ�ι���
MnCl2+Cl2��+2H2OС���淴Ӧ�Ľ��У�����Ũ�ȱ�С��ϡ����Ͷ������̲���ӦCl2+2OH�C=Cl�C+ClO�C+H2O28.6g��ͷ�ι���
�����������������I�����⿼��ʵ������ȡ��������1������������̺�Ũ������ȷ�Ӧ�õ�������(2)����������Ũ���ᷴӦ��������������������ϡ�����Ӧ��(3)��������������������Һ��II�����⿼���ù�������һ�����ʵ���Ũ�ȵ���Һ��ʵ�鲽�衢����480mL��Һ��Ҫ500mL������ƿ������������C(��)V(��)=C(��)V(��)������
������(1)Բ����ƿ�ж������̺�Ũ���ᷢ����Ӧ���������ķ�Ӧ����ʽ��MnO2+4HCl�� Ũ��![]() MnCl2+Cl2��+2H2O ��(2) ����������Ũ���ᷴӦ��������������������ϡ�����Ӧ������������������20 mL 12 mol��L��1�������ϼ��ȣ���������Ļӷ����������ϡ���ٷ�Ӧ������������ʣ�࣬���Գ�ַ�Ӧ�����ɵ���������С��0.06 mol��(3)�����������������������Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽ��Cl2+2OH�C =Cl�C+ClO�C+H2O��
MnCl2+Cl2��+2H2O ��(2) ����������Ũ���ᷴӦ��������������������ϡ�����Ӧ������������������20 mL 12 mol��L��1�������ϼ��ȣ���������Ļӷ����������ϡ���ٷ�Ӧ������������ʣ�࣬���Գ�ַ�Ӧ�����ɵ���������С��0.06 mol��(3)�����������������������Ȼ��ơ��������ƺ�ˮ�����ӷ���ʽ��Cl2+2OH�C =Cl�C+ClO�C+H2O��
II����Na2CO3��10H2O���壬����0.2 mol��L��1��Na2CO3��Һ480 mL��
��1������0.2 mol��L��1��Na2CO3��Һ480 mL����Ҫ500mL������ƿ��Ӧ��ȡNa2CO3��10H2O�����������0.2 mol��L��1��0.5L��286g/mol=28.6g������ʱ��������ƿ�м�ˮ����1��2cmʱ�����ý�ͷ�ιܼ�ˮ���̶ȣ��Ӹ�ҡ�ȣ�
��2����Na2CO3��10H2O����ʧȥ�˲��ֽᾧˮ��̼��������ƫ��������ҺŨ��ƫ�ߣ��������������������ij���������������(ʹ������)����������С��28.6g��������ҺŨ��ƫС������̼���ƾ��岻�������л����Ȼ��ƣ�̼��������ƫС��������ҺŨ��ƫС����������ƿδ������ʹ�ã���Ӱ�졣 ���� ��������������ҺŨ��ƫ�ߵ�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���Է�Һ�к���Fe2+��Cu2+��Ba2+���ֽ������ӣ���ͬѧ��������з����Է�Һ���д����������Լ����Թ��������Ի��ս���������������

��ش�
��1������a�к��еĵ�����_________��
��2������b�Ļ�ѧʽ��__________��
��3������c�Ļ�ѧʽ��__________��
��4����ҺA��H2O2��Һ�����������·�Ӧ�����ӷ���ʽ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(I)A��B��C��D��E��F��G��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӣ�BԪ��ԭ�������������Ǵ�����������2����D�ǵؿ��к�������Ԫ�أ�E�Ƕ������н�������ǿ��Ԫ�أ�F��Gλ�����ڣ�G��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�ء�
���û�ѧ����ش�
(1)A��D�γɵ�18���ӵĻ�������FD2��������һ��ǿ�ᣬд����ǿ����ʽ����ˮ��Һ�ĵ��뷽��ʽΪ��________________��
(2)�õ���ʽ��ʾ������E2D���γɹ��̣�________________��
(3)��l0lkPa��25���£�14g��̬B2A4��D2����ȫȼ�գ��ų�QkJ��������B2A4��ȼ���ȵ��Ȼ�ѧ����ʽΪ��________________��
(��)A��B��C��X��Ϊ�����Ĵ��������֮��������ת����ϵ(����Ʒ����ȥ)
![]()
��ͬ��
(4)��X��ǿ�����Ե��ʣ���A��������___________��
a.H,S b. NH3c.Na d.Zn e.CH3CH2OH
(5)��X�ǽ������ʣ���C��ˮ��Һ�е���AgNO3��Һ������������ϡHNO3�İ�ɫ��������C�Ļ�ѧʽΪ________________��
(6)��A��B��CΪ��ij����Ԫ�ص��������XΪǿ����ʣ�A��Һ��C��Һ��Ӧ����B����B�Ļ�ѧʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���Xmol N2 ��Ymol H2�Ļ������ͨ��һ���̶��ݻ����ܱ������У��������·�Ӧ��N2(g)��3N2(g)![]() 2NH3(g)
2NH3(g)
������Ӧ���е�tʱ�̣�n(N2)=10mol��n(NH3)=4mol������X��ֵ��
����Ӧ�ﵽƽ��ʱ�������������Ϊ672L(��״����)������NH3���������Ϊ20%������N2��ת���ʺ�Y��ֵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ת���У�A��һ����ʽ�Σ�D����Է���������C����Է���������16��E���ᣬ��X������ǿ�ỹ��ǿ��ʱ���������µ�ת����ϵ��
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
��X��ǿ��ʱ��A��B��C��D��E����ͬһ��Ԫ�أ���X��ǿ��ʱ��A��B��C��D��E��������ͬһ��Ԫ�ء���ش�
(1)A��________�� Z��________��
(2)��X��ǿ��ʱ��д��B����C�Ļ�ѧ����ʽ��___________________��
(3)��X��ǿ��ʱ��E��________��д��E��ͭ��Ӧ����C�Ļ�ѧ����ʽ��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б仯�������ȷ�Ӧ������ ��
��Һ̬ˮ�����ڽ��������ȱ�Ϊ��ɫ��ĩ��Ũ����ϡ�͢�����طֽ�����������ʯ�Ҹ�ˮ��Ӧ������ʯ��
A���٢ܢ� B���٢ڢ� C���ڢ� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���д������
A. ���ݶԽ��߹�������������ʾ���������
B. [Cu(H2O)4]2+��Cu�ṩ�չ����H2O��O�ṩ�¶Ե����γ���λ��
C. Ԫ�ص縺��Խ���ԭ�ӣ��������ӵ�����Խǿ
D. ���Է��ӻ�Ϊ�������ǵ�����û������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������ƣ���һ�������������м��ȣ��ڶ�������������(����)�г�ַ�Ӧ��������˵����ȷ����(������)
A. ��һ����ʧȥ���Ӷ�
B. ������ʧȥ����һ����
C. �ڶ����Ƶķ�Ӧ�����������
D. �ڶ����Ƶķ�Ӧ�����ڿ����и��ȶ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com