
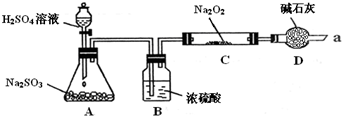
��16�֣�ijͬѧ��ͨ����ͼװ��ʵ�顢̽��SO2��Na2O2��Ӧ�IJ��
���г�װ������ȥ��װ�õ����������ã�
��װ��B������������ˮ������SO2���壬��ԭ���ǣ������ӷ���ʽ��ʾ��
��
װ��D�������չ�����SO2��������Ⱦ�����⣬����������
��
��ijͬѧ��ͨ����ͼʵ��װ�ã����鷴Ӧ���Ƿ���O2����ʱ����ʵ����������ǣ�
�� ��
�����ô����ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ��
��C�й������������¼��裺
����1��ֻ��Na2SO3
����2��ֻ��Na2SO4
����3�� ��
��1��������2������д����������Ӧ�Ļ�ѧ����ʽ
��
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺
�ó����ۣ�������Na2SO4��
�÷����Ƿ���� ����ǡ�����
��3�����ʵ����֤����3��ʵ�鲽�����£�
| ʵ�鲽�� | ʵ������ |
| �ٵμ��������ϡ���� | �����ݼ�����ζ���� |
| �ڵμ���������BaCl2��Һ | ������ɫ������ |
| ��ȡ����C�й���������Թ��У���������������ˮ�ܽ� |
|
������3��������ȷ��ʵ�����˳����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
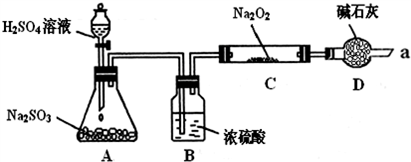

| ʵ�鲽�� | Ԥ������ͽ��� |
| ��ȡ����C�й���������Թ��У���������������ˮ�ܽ� | |
| �� | |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
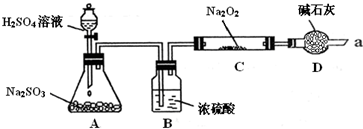
| ʵ����� | Ԥ������ | �� �� |
| ����һ��ȡ����C�й���������Թ��У���������������ˮ�ܽ⣬�ֱ�ȡ������A��B�Թ��� | ������ȫ�ܽ⣬�õ���ɫ����Һ | / |
| �������ȡ�Թ�A������Һ�м��� |
����1 �����3��������û�и����������2������ | |
| ��������ȡ�Թ�B���������ȼ��� �ټ��� |
���� �ټ����Լ�����������ɫ������ |
����3���������ް�ɫ�������������1������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ������ѧ�ڵڶ���ͳ����ѧ�Ծ� ���ͣ�ʵ����
��16�֣���ijͬѧ��ͨ����ͼװ�ã��г�װ������ȥ��ʵ�飬̽��SO2��Na2O2��Ӧ�IJ��
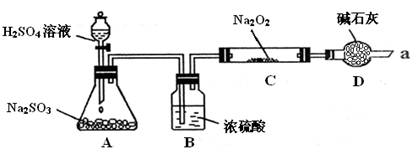
��װ��B������ ��
װ��D������ ��
����μ��鷴Ӧ���Ƿ���O2����
��
��C�й������������¼��裺
����1��ֻ��Na2SO3
����2��ֻ��Na2SO4
����3�� ��
��1������2�ķ�Ӧ����ʽΪ ��
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺

�ó����ۣ�������Na2SO4��
�÷����Ƿ���� ����ǡ����������� ��
��3�����ʵ����֤����3��ȡ����C�й���������Թ��У���������������ˮ�ܽ⣬ �������3��������д���������Ӧ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��㶫ʡ����9���¿���ѧ���� ���ͣ�ʵ����
��16�֣�ijͬѧ��ͨ����ͼװ��ʵ�顢̽��SO2��Na2O2��Ӧ�IJ��
���г�װ������ȥ��װ�õ����������ã�
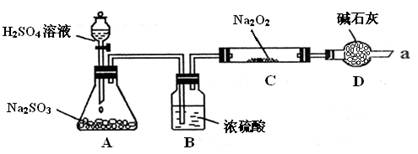
����װ��B������������ˮ������SO2���壬��ԭ���ǣ������ӷ���ʽ��ʾ��
��
װ��D�������չ�����SO2��������Ⱦ�����⣬����������
��
����ijͬѧ��ͨ����ͼʵ��װ�ã����鷴Ӧ���Ƿ���O2����ʱ����ʵ����������ǣ�
�� ��
�����ô����ǵ�ľ����������ܿ�a���۲����Ƿ�ȼ��
������C�й������������¼��裺
����1��ֻ��Na2SO3
����2��ֻ��Na2SO4
����3�� ��
��1��������2������д����������Ӧ�Ļ�ѧ����ʽ
��
��2����Na2O2��Ӧ��ȫ��Ϊȷ��C�й������ijɷ֣����������ʵ�飺

�ó����ۣ�������Na2SO4��
�÷����Ƿ���� ����ǡ�����
��3�����ʵ����֤����3��ʵ�鲽�����£�
|
ʵ�鲽�� |
ʵ������ |
|
�ٵμ��������ϡ���� |
�����ݼ�����ζ���� |
|
�ڵμ���������BaCl2��Һ |
������ɫ������ |
|
��ȡ����C�й���������Թ��У���������������ˮ�ܽ� |
|
������3��������ȷ��ʵ�����˳����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com