���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������
��������̼���ȵķ�������SrCO
3 38.40%��SrO12.62%��CaCO
3 38.27%��BaCO
3 2.54%���������������������8.17%����ϡ���ᷴӦ�����˺���Һ��Ӧ�ú���Sr��NO
3��
2��Ca��NO
3��
2��Ba��NO
3��
2���ɱ��е����ݿ��Կ�����Sr��NO
3��
2���ܽ�������¶ȵ����߱仯��������Ƶ��ܽ�����¶ȱ仯�ϴ���˿�ͨ�������ᾧ�����ȹ��ˣ�ϴ�ӣ�����������ȴֲ�Ʒ��
��1��Ũ�������������Ϊ65%���ܶ�Ϊ1.4g/cm
3��Ҫ����30%ϡ����500mL��v��Ũ���ᣩ���ѣ�Ũ���ᣩ��Ũ�������������=m��ϡ���ᣩ��ϡ���������������m��ϡ���ᣩ=v��ϡ���ᣩ���ѣ�ϡ���ᣩ���ʻ���Ҫ����ϡ������ܶȣ������ƹ����в�ʹ����ƽ�������Ҫ���������Ϊv��Ũ���ᣩ��v��ϡ���ᣩ����ʵ����ȡ��Ӧ�ǣ�Ũ���������ˮ�����������ʹ�õ���������Ͳ���ձ�����������
��2���ɱ��е����ݿ��Կ�����Sr��NO
3��
2���ܽ�������¶ȵ����߱仯��������Ƶ��ܽ�����¶ȱ仯�ϴ���˿�ͨ���ɽ�ȡ��õ��Ļ�����Ʊ������ȴ�Ʒ��ʵ�鲽������Ϊ�������ᾧ�����ȹ��ˣ�ϴ�ӣ�����õ���
��3���ٷ����ζ����̿�֪����Ʒ��Һ��ɫ������̼���Ƴ�����ȫ���Ե����̪��Һָʾ�յ㣬�������һ����Һ�ʺ�ɫ��������ڲ���ɫ�������ζ�ǰ��Ʒ��Ca��NO
3��
2û�г����������ı���Һ̼���ƣ����ݵζ���������c������Һ��=
�����ı�Һ�࣬���ⶨ�������ȴ��Ȼ�ƫ�ߣ�
����֪��������������л��ܼ�A�У�ȡ5.39g��������Ʒ�������������л��ܼ�A�������ˡ�ϴ�ӡ������ʣ�����5.26g��ʣ�����Ϊ���ᱵ�������ȣ��������Ⱥ����ᱵ�����ʵ���Ϊx��y������������Ϊ5.26g������212x+261y=5.26�٣���̼�����Ӧ��ֻ�б����Ӻ������ӣ�Sr
2++CO
32-��SrCO
3����Ba
2++CO
32-��BaCO
3��������Ũ��Ϊ0.107mol/L��̼������Һ22.98mL�����ʵ���Ϊ0.00246mol������x+y=0.0246�ڣ��ɢ٢�ʽ��m�������ȣ�=212x=5.02g�������ȵ���������=
��100%=0.93�����ζ�ǰ��Ʒ��Ca��NO
3��
2û�г������ζ���Һʱ����̼������Һ�࣬�������������ʵ���ƫ�����ⶨ�������ȴ��Ƚ���ƫ�ߣ�
���
�⣺��̼���ȵķ�������SrCO
3 38.40%��SrO12.62%��CaCO
3 38.27%��BaCO
3 2.54%���������������������8.17%����ϡ���ᷴӦ�����˺���Һ��Ӧ�ú���Sr��NO
3��
2��Ca��NO
3��
2��Ba��NO
3��
2���ɱ��е����ݿ��Կ�����Sr��NO
3��
2���ܽ�������¶ȵ����߱仯��������Ƶ��ܽ�����¶ȱ仯�ϴ���˿�ͨ�������ᾧ�����ȹ��ˣ�ϴ�ӣ�����������ȴֲ�Ʒ��
��1��Ũ�������������Ϊ65%���ܶ�Ϊ1.4g/cm
3��Ҫ����30%ϡ����500mL��v��Ũ���ᣩ���ѣ�Ũ���ᣩ��Ũ�������������=m��ϡ���ᣩ��ϡ���������������m��ϡ���ᣩ=v��ϡ���ᣩ���ѣ�ϡ���ᣩ���ʻ���Ҫ����ϡ������ܶȣ������ƹ����в�ʹ����ƽ�������Ҫ���������Ϊv��Ũ���ᣩ��v��ϡ���ᣩ����ʵ����ȡ��Ӧ�ǣ�Ũ��������ˮ�����������ʹ�õ���������Ͳ���ձ�����������
�ʴ�Ϊ��30%ϡ������ܶȣ�Ũ���������ˮ���������Ͳ���ձ�����������
��2���ɱ��е����ݿ��Կ�����Sr��NO
3��
2���ܽ�������¶ȵ����߱仯��������Ƶ��ܽ�����¶ȱ仯�ϴ���˿�ͨ���ɽ�ȡ��õ��Ļ�����Ʊ������ȴ�Ʒ��ʵ�鲽������Ϊ�������ᾧ�����ȹ��ˣ�ϴ�ӣ�����õ���
�ʴ�Ϊ�������ᾧ�����ȹ��ˣ�
��3���ٷ����ζ����̿�֪����Ʒ��Һ��ɫ������̼���Ƴ�����ȫ���Ե����̪��Һָʾ�յ㣬�������һ����Һ�ʺ�ɫ��������ڲ���ɫ�������ζ�ǰ��Ʒ��Ca��NO
3��
2û�г����������ı���Һ̼���ƣ����ݵζ���������c������Һ��=
�����ı�Һ�࣬���ⶨ�������ȴ��Ȼ�ƫ�ߣ�
�ʴ�Ϊ����̪����Һ��Ϊ��ɫ����30s�ڲ���ɫ��
����֪��������������л��ܼ�A�У�ȡ5.39g��������Ʒ�������������л��ܼ�A�������ˡ�ϴ�ӡ������ʣ�����5.26g��ʣ�����Ϊ���ᱵ�������ȣ��������Ⱥ����ᱵ�����ʵ���Ϊx��y������������Ϊ5.26g������212x+261y=5.26�٣���̼�����Ӧ��ֻ�б����Ӻ������ӣ�Sr
2++CO
32-��SrCO
3����Ba
2++CO
32-��BaCO
3��������Ũ��Ϊ0.107mol/L��̼������Һ22.98mL�����ʵ���Ϊ0.00246mol������x+y=0.0246�ڣ��ɢ٢�ʽ��m�������ȣ�=212x=5.02g�������ȵ���������=
��100%=0.93�����ζ�ǰ��Ʒ��Ca��NO
3��
2û�г������ζ���Һʱ����̼������Һ�࣬�������������ʵ���ƫ�����ⶨ�������ȴ��Ƚ���ƫ�ߣ�
�ʴ�Ϊ��0.93��ƫ��
���������⿼���������Ʊ�ʵ�鷽���ķ����жϣ�������������̣���ѧ��Ӧ���ʵ�Ӱ�����ط������ζ�ʵ��IJ����ָʾ��ѡ��������Ӧ�ã�

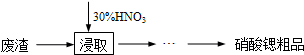



 ��������Ȼ�̼��Һ��Ӧ���ɲ�������� �������������칹����������
��������Ȼ�̼��Һ��Ӧ���ɲ�������� �������������칹����������