
BaCl2��xH2O�нᾧˮ��Ŀ��ͨ����������ȷ����
�ٳ�ȡ1.222 g��Ʒ������С�ձ��У���������ϡ���ᣬ�����ܽ⣬�߽���ߵμ�ϡ���ᵽ������ȫ�����ã�
�ڹ��˲�ϴ�ӳ�����
�۽�ʢ�г�������ֽ����ɲ��������գ�ת�����¯�У��������������أ��Ƶó�������Ϊ1.165 g��
�ش��������⣺
(1)�ڲ������У���Ҫ�Ⱥ���ϡ�����________ϴ�ӳ���������������������Ƿ�ϴ���ķ�����__________________________________________________________��
(2)����BaCl2��xH2O�е�x��________(Ҫ��д���������)��
(3)�������У����������������¶ȹ��ߣ����ܻ��в��ֳ�������ֽ�е�̼��ԭΪBaS����ʹx�IJⶨ���________(�ƫ�͡���ƫ�ߡ����䡱)��
(1)����ˮ��ȡˮϴҺ���Թ��У�����ϡ�����ữ���μ�AgNO3��Һ�����ް�ɫ���dz��֣������Cl���Ѿ�ϴ����
(2)��Ʒ��BaCl2�����ʵ���Ϊn(BaCl2)��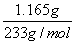 ��5.000��10��3 mol
��5.000��10��3 mol
m(BaCl2)��5.000��10��3 mol��208 g/mol��1.040 g
n(H2O)��n(BaCl2)��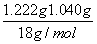 ��(5.000��10��3 mol)��2.02��2
��(5.000��10��3 mol)��2.02��2
(3)ƫ��
[����] (1)ϴ�ӳ���Ҫ������ˮ��������ϴ�Ӻ�ϴ��Һ�в���Cl�����Ѿ�ϴ����(3)�����Ѿ��������ᱵ����ԭΪBaS�������������С��������һ������ô����õ�ˮ������ƫ��x���ݽ�ƫ�ߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Ȼ�ѧ����ʽ�����ӷ���ʽ�У���ȷ���ǣ�
A.����ı�ȼ����Ϊ-890.3kJ��mol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��
CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H=-890.3kJ��mol-1
B. 500�桢30MPa�£���0.5mol N2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3(g)������19.3kJ�����Ȼ�ѧ����ʽΪ��
 ��H=-38.6kJ��
��H=-38.6kJ�� mol-1
mol-1
C. �Ȼ�þ��Һ�백ˮ��Ӧ��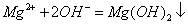
D. ����������NaOH��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Fe��OH��3+3H+=Fe2++3H2O
��������������Ҫ�ɷ�Ϊ Fe2O3��Fe3O4��FeO��SiO2�ȣ�����������Ĺ�ҵ���������ۺ����öԻ�������������ʵ���塣���������������Ʊ�����Ȳ�Ʒ��ʵ���������£�

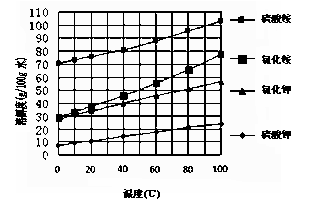 ��֪�����ε��ܽ�����¶ȱ仯��
��֪�����ε��ܽ�����¶ȱ仯��
��������ͼ��ʾ��
�ش��������⣺
��1����������˺���Һ�еĽ���������
��______________��
��2������ FeCO3���ɲ�Ʒ I�Ļ�ѧ��Ӧ
����ʽΪ _______��ʵ���ҽ������ղ�����
���������˾ƾ���ơ������ǡ����żܡ���
�����⣬����__________��
��3����Ʒ��Ļ�ѧʽΪ _______________��Ϊ�˻�ò�Ʒ���� ��NH4��2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǣ� ��
��4�������Ʒ II���Ƿ����Ȼ������ʵ�ʵ������ǣ�ȡ������Ʒ�����Թ��������Һ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���� �� ��
A��NaCl��Һ���磬����NaCl��Һ�ǵ���ʣ�
B��Cu�ܵ��磬����Cu�ǵ���ʣ�
C��SO3����ˮ�ܵ��磬����SO3�ǵ���ʣ�
D��Һ̬�ƾ����ƾ���ˮ��Һ�����磬���Ծƾ��Ƿǵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤��������ֵ������˵����ȷ����(����)
A�������£�0.2 mol Fe������ˮ������Ӧ�����ɵ�H2������ĿΪ0.3 NA
B�������£�1 L pH��13��NaOH��Һ�У���ˮ�����OH����ĿΪ0.1NA
C������ȼ�ϵ����������22.4 L(��״��)����ʱ����·��ͨ���ĵ�����ĿΪ2NA
D��5NH4NO3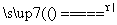 2HNO3��4N2����9H2O��Ӧ�У�����28 g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
2HNO3��4N2����9H2O��Ӧ�У�����28 g N2ʱ��ת�Ƶĵ�����ĿΪ3.75NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)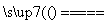 Na2S2O3(aq)
Na2S2O3(aq)
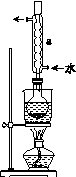
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O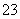 ��I2===S4O
��I2===S4O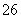 ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO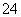 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
BaCl2��xH2O�нᾧˮ��Ŀ��ͨ����������ȷ����
�ٳ�ȡ1.222g��Ʒ������С�ձ��У���������ϡ���ᣬ�����ܽ⣬�߽���ߵμ�ϡ���ᵽ������ȫ�����ã�
�� ���˲�ϴ�ӳ�����
���˲�ϴ�ӳ�����
�۽�ʢ�г�������ֽ����ɲ��������գ�ת�����¯�У��������յ����أ��Ƶó�������Ϊ1.165g��
�ش��������⣺
��1���ڲ������У���Ҫ�Ⱥ���ϡ����� ϴ�ӳ���������������������Ƿ�ϴ���ķ����� ��
��2������BaCl2��xH2O�е�x= ��Ҫ��д��������̣���
��3���������У����������������¶ȹ��ߣ����ܻ��в��ֳ�������ֽ�е�̼��ԭΪBaS����ʹx�IJⶨ��� ���ƫ�͡�����ƫ�ߡ����䡱����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA��ʾ�����ӵ�����������������ȷ����
A�������ʵ�����N2��CO������������ΪNA
B��1.7g H2O2�к��еĵ�����Ϊ0.9 NA
C��1mol Na2O2 �����к���������Ϊ4 NA
D����״���£�2.24L��������������Ϊ0.1 NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ָ�����ӵ� ��Ŀ����6.02��1023���ǣ�������
��Ŀ����6.02��1023���ǣ�������
A.2 g��ˮ��D2O��DΪ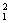 H���к��е�������
H����������
B.0.1 mol F���к��еĵ�����
C.��״����11.2 L N2��NO��������е�ԭ����
D.1 L 1 mol��L��1Na2SO4��Һ�е�Na+������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com