
ΓΨΧβΡΩΓΩν―±Μ≥ΤΈΣΦΧΧζΓΔ¬Ν÷°ΚσΒΡΒΎ»ΐΫπ τΘ§«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)ΫπΚλ ·(TiO2) «ν―ΒΡ÷ς“ΣΩσΈο÷°“ΜΘ§ΜυΧ§Ti‘≠Ή”Φέ≤ψΒγΉ”ΒΡ≈≈≤ΦΆΦΈΣ_________Θ§ΜυΧ§O‘≠Ή”ΒγΉ”’ΦΨίΉνΗΏΡήΦΕΒΡΒγΉ”‘Τ¬÷άΣΆΦΈΣ __________–ΈΓΘ
(2)“‘TiO2ΈΣ‘≠ΝœΩ…÷ΤΒΟTiCl4Θ§TiCl4ΒΡ»έΓΔΖ–ΒψΖ÷±πΈΣ205KΓΔ409KΘ§ΨυΗΏ”ΎΫαΙΙ”κΤδœύΥΤΒΡCCl4Θ§÷ς“Σ‘≠“ρ « __________________ΓΘ
(3)TiCl4Ω…»ή”Ύ≈®―ΈΥαΒΟH2[TiCl6]Θ§œρ»ή“Κ÷–Φ”»κNH4Cl≈®»ή“ΚΩ…Έω≥ωΜΤ…ΪΒΡ(NH4)2[TiCl6]ΨßΧεΓΘΗΟΨßΧε÷–ΈΔΙέΝΘΉ”÷°ΦδΒΡΉς”ΟΝΠ”– ________ΓΘ
AΘ°άκΉ”Φϋ BΘ°Ι≤ΦέΦϋ CΘ°Ζ÷Ή”ΦδΉς”ΟΝΠ DΘ°«βΦϋ EΘ°ΖΕΒ¬ΜΣΝΠ
(4)TiCl4Ω…”κCH3CH2OHΓΔHCHOΓΔCH3OCH3Β»”–Μζ–ΓΖ÷Ή”–Έ≥…Φ”ΚœΈοΓΘ…œ ω»ΐ÷÷–ΓΖ÷Ή”÷–C‘≠Ή”ΒΡVSEPRΡΘ–Ά≤ΜΆ§”ΎΤδΥϊΖ÷Ή”ΒΡ « _____Θ§ΗΟΖ÷Ή”÷–CΒΡΙλΒά‘”Μ·άύ–ΆΈΣ________ ΓΘ
(5)TiO2”κBaCO3“ΜΤπ»έ»ΎΩ…÷ΤΒΟν―Υα±ΒΓΘ
ΔΌBaCO3÷–“θάκΉ”ΒΡΝΔΧεΙΙ–ΆΈΣ ________ΓΘ
ΔΎΨ≠X…δœΏΖ÷ΈωΦχΕ®Θ§ν―Υα±ΒΒΡΨßΑϊΫαΙΙ»γœ¬ΆΦΥυ ΨΘ®Ti4+ΓΔBa2+Ψυ”κO2Θ≠œύΫ”¥ΞΘ©Θ§‘ρν―Υα±ΒΒΡΜ·―ß ΫΈΣ _________ΓΘ“―÷ΣΨßΑϊ±Ώ≥ΛΈΣa pmΘ§O2Θ≠ΒΡΑκΨΕΈΣb pmΘ§‘ρTi4+ΓΔBa2+ΒΡΑκΨΕΖ÷±πΈΣ____________pmΓΔ___________pmΓΘ

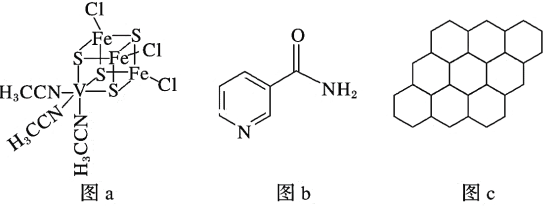
ΓΨ¥πΑΗΓΩ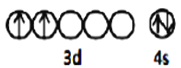 ―ΤΝε–Ά TiCl4ΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ”ΎCCl4Θ§Ζ÷Ή”ΦδΉς”ΟΝΠΗϋ¥σ AB HCHO sp2 ΤΫΟφ»ΐΫ«–Έ BaTiO3
―ΤΝε–Ά TiCl4ΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ”ΎCCl4Θ§Ζ÷Ή”ΦδΉς”ΟΝΠΗϋ¥σ AB HCHO sp2 ΤΫΟφ»ΐΫ«–Έ BaTiO3 ![]()
![]()
ΓΨΫβΈωΓΩ
(1)ΗυΨί‘ΣΥΊΚΥΆβΒγΉ”≈≈≤ΦΙφ¬… ι–¥ΒγΉ”≈≈≤Φ ΫΘ§ΗυΨίΒγΉ”≈≈≤Φ Ϋ≈–ΕœΉνΗΏΡήΦΕΦΑΒγΉ”‘Τ¬÷άΣΘΜ
(2)Ζ÷Ή”ΨßΧεΒΡ»έΖ–Βψ”κΖ÷Ή”ΦδΉς”ΟΝΠ”–ΙΊΘ§ΗυΨίœύΕ‘Ζ÷Ή”÷ ΝΩΖ÷Έω≈–ΕœΘΜ
(3)ΫαΚœΨßΧεΉΣΜ·Ζ¥”ΠΙΐ≥ΧΘ§ΚΆΈο÷ άύ±π≈–ΕœΖ÷ΈωΜ·―ßΦϋΒΡ÷÷άύΘΜ
(4)ΗυΨί”–ΜζΈο÷–ΧΦ‘≠Ή”ΒΡ≥…ΦϋΖΫ ΫΘ§≈–ΕœΩ’ΦδΙΙ–ΆΘ§ΫχΕχ≈–ΕœΧΦ‘≠Ή”‘”Μ·ΖΫ ΫΘΜ
(5)ΔΌ”Π”Ο‘”Μ·ΙλΒάάμ¬έΦΤΥψ÷––Ρ‘≠Ή”ΒΡΦέΒγΉ”Ε‘ ΐ»ΖΕ®‘”Μ·ΖΫ ΫΖ÷Έω»ΖΕ®ΝΔΧεΙΙ–ΆΘΜ
ΔΎΫαΚœΨßΑϊΆΦ ΨΦΤΥψΨßΑϊ÷–Ης‘≠Ή”ΒΡΗω ΐ ι–¥ΤδΖ÷Ή” ΫΘ§‘ΌΫαΚœΨßΑϊΈΔΝΘΒΡœύΜΞΈΜ÷ΟΙΊœΒΦΤΥψΈΔΝΘΒΡΑκΨΕΓΘ
(1) TiΈΣ38Κ≈‘ΣΥΊΘ§ΜυΧ§ν―‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣΘΚ[Ar]3d24s2Θ§‘ρΦέ≤ψΒγΉ”≈≈≤ΦΆΦΈΣ![]() ΘΜΜυΧ§O‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p4Θ§ΉνΗΏΡήΦΕΈΣpΘ§ΤδΒγΉ”‘Τ¬÷άΣΈΣ―ΤΝε–ΆΘΜ
ΘΜΜυΧ§O‘≠Ή”ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ1s22s22p4Θ§ΉνΗΏΡήΦΕΈΣpΘ§ΤδΒγΉ”‘Τ¬÷άΣΈΣ―ΤΝε–ΆΘΜ
(2) TiCl4ΒΡ»έΓΔΖ–ΒψΖ÷±πΈΣ205KΓΔ409KΘ§”κCCl4ΫαΙΙœύΥΤΘ§ΕΦ τ”ΎΖ÷Ή”ΨßΧεΘ§Ζ÷Ή”ΨßΧεΒΡ»έΖ–Βψ”κΖ÷Ή”ΦδΉς”ΟΝΠ”–ΙΊΘ§œύΕ‘Ζ÷Ή”÷ ΝΩ‘Ϋ¥σΘ§Ζ÷Ή”ΦδΉς”ΟΝΠ‘Ϋ¥σΘ§»έΖ–Βψ‘ΫΗΏΘ§TiCl4ΒΡœύΕ‘Ζ÷Ή”÷ ΝΩ¥σ”ΎCCl4Θ§Ζ÷Ή”ΦδΉς”ΟΝΠΗϋ¥σΘΜ
(3)ΗυΨίΉΣΜ·Ιΐ≥ΧTiCl4Ω…»ή”Ύ≈®―ΈΥαΒΟH2[TiCl6]Θ§Ω…Ω¥Ήω–Έ≥…“Μ÷÷ΥαΘ§Υυ”–ΒΡΥαΕΦ τ”ΎΙ≤ΦέΜ·ΚœΈοΘ§œρ»ή“Κ÷–Φ”»κNH4Cl≈®»ή“ΚΩ…Έω≥ωΜΤ…ΪΒΡ(NH4)2[TiCl6]ΨßΧεΘ§Ω…Ω¥ΉωΥαΗζ―ΈΖ¥”Π…ζ≥…(NH4)2[TiCl6]Θ§≤ζΈο÷–Κ§”–οßΗυάκΉ”Θ§ΗυΨί“‘…œΖ÷ΈωΘ§(NH4)2[TiCl6]ΨßΧε÷–Κ§”–Ι≤ΦέΦϋΚΆάκΉ”ΦϋΘ§Ι ¥πΑΗ―ΓABΘΜ
(4) CH3CH2OHΚΆCH3OCH3÷–ΒΡΧΦ‘≠Ή”ΕΦ «“‘ΒΞΦϋ–Έ Ϋ≥…ΦϋΘ§ΫαΙΙ”κΦΉΆιœύΥΤΘ§ΕΦ «ΥΡΟφΧεΫαΙΙΘ§HCHOΒΡΧΦ‘≠Ή”Κ§”–ΧΦ―θΥΪΦϋΘ§Ζ÷Ή”÷–Υυ”–‘ΎΆ§“ΜΤΫΟφΘ§ΈΣΤΫΟφ»ΐΫ«–ΈΘ§ΗυΨίΙΙ–ΆΩ…÷ΣΘ§»ΐΗωΖ÷Ή”÷–C‘≠Ή”ΒΡVSEPRΡΘ–Ά≤ΜΆ§”ΎΤδΥϊΖ÷Ή”ΒΡ «HCHOΘ§ΗυΨίΙΙ–ΆΩ…ΒΟΘ§ΗΟΖ÷Ή”÷–CΒΡΙλΒά‘”Μ·άύ–ΆΈΣsp2‘”Μ·ΘΜ
(5)ΔΌBaCO3÷–“θάκΉ”ΈΣCO32-Θ§÷––Ρ‘≠Ή”ΈΣΧΦ‘≠Ή”Θ§ΤδΦέ≤ψΒγΉ”Ε‘ ΐ=3+![]() =3Θ§ΧΦ‘≠Ή”ΈΣsp2‘”Μ·Θ§ΗΟ“θάκΉ””…4Ηω‘≠Ή”ΙΙ≥…Θ§‘ρΝΔΧεΙΙ–ΆΈΣΤΫΟφ»ΐΫ«–ΈΘΜ
=3Θ§ΧΦ‘≠Ή”ΈΣsp2‘”Μ·Θ§ΗΟ“θάκΉ””…4Ηω‘≠Ή”ΙΙ≥…Θ§‘ρΝΔΧεΙΙ–ΆΈΣΤΫΟφ»ΐΫ«–ΈΘΜ
ΔΎΗυΨίΨßΑϊΆΦ ΨΘ§TiΈΜ”ΎΨßΑϊΒΡΕΞΒψΘ§TiΒΡ ΐΡΩ=8ΓΝ![]() =1Θ§Ba‘≠Ή”ΈΜ”ΎΨßΑϊΒΡΡΎ≤ΩΘ§ ΐΡΩΈΣ1Θ§Ζ÷ΈωΦΤΥψΖ÷Ή” ΫΚΆΝΘΉ”ΑκΨΕΘΜO‘≠Ή”ΈΜ”ΎΨßΑϊΒΡάβ…œΘ§Τδ ΐΡΩ=12ΓΝ
=1Θ§Ba‘≠Ή”ΈΜ”ΎΨßΑϊΒΡΡΎ≤ΩΘ§ ΐΡΩΈΣ1Θ§Ζ÷ΈωΦΤΥψΖ÷Ή” ΫΚΆΝΘΉ”ΑκΨΕΘΜO‘≠Ή”ΈΜ”ΎΨßΑϊΒΡάβ…œΘ§Τδ ΐΡΩ=12ΓΝ![]() =3Θ§‘ρ‘ρν―Υα±ΒΒΡΜ·―ß ΫΈΣBaTiO3ΘΜ“―÷ΣΨßΑϊ±Ώ≥ΛΈΣa pmΘ§O2Θ≠ΒΡΑκΨΕΈΣbpmΘ§ΗυΨίΆΦ ΨΘ§ΨßΑϊ±Ώ≥Λ= 2r(Ti4+)+2r(O2-)=apmΘ§‘ρr(Ti4+)=
=3Θ§‘ρ‘ρν―Υα±ΒΒΡΜ·―ß ΫΈΣBaTiO3ΘΜ“―÷ΣΨßΑϊ±Ώ≥ΛΈΣa pmΘ§O2Θ≠ΒΡΑκΨΕΈΣbpmΘ§ΗυΨίΆΦ ΨΘ§ΨßΑϊ±Ώ≥Λ= 2r(Ti4+)+2r(O2-)=apmΘ§‘ρr(Ti4+)=![]() pmΘΜΨßΑϊΟφΕ‘Ϋ«œΏΒΡ≥ΛΕ»=2r(O2-)+2r(Ba2+)=
pmΘΜΨßΑϊΟφΕ‘Ϋ«œΏΒΡ≥ΛΕ»=2r(O2-)+2r(Ba2+)=![]() a pmΘ§r(Ba2+)=
a pmΘ§r(Ba2+)=![]() pmΓΘ
pmΓΘ



| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΗςΉι»»Μ·―ßΖΫ≥Χ Ϋ÷–Θ§Μ·―ßΖ¥”ΠΒΡΓςH «Α’Ώ–Γ”ΎΚσ’ΏΒΡ «
ΔΌCΘ®sΘ©+O2Θ®gΘ©®TCO2Θ®gΘ©ΓςH1 CΘ®sΘ©+![]() O2®TCOΘ®gΘ©ΓςH2
O2®TCOΘ®gΘ©ΓςH2
ΔΎSΘ®gΘ©+O2Θ®gΘ©®TSO2Θ®gΘ©ΓςH3 SΘ®sΘ©+O2Θ®gΘ©®TSO2Θ®gΘ©ΓςH4
Δέ2H2Θ®gΘ©+O2Θ®gΘ©®T2H2OΘ®lΘ©ΓςH5 H2Θ®gΘ©+![]() O2Θ®gΘ©®TH2OΘ®lΘ©ΓςH6
O2Θ®gΘ©®TH2OΘ®lΘ©ΓςH6
ΔήCaCO3Θ®sΘ©®TCaOΘ®sΘ©+CO2Θ®gΘ©ΓςH7 CaOΘ®sΘ©+H2OΘ®lΘ©®TCaΘ®OHΘ©2Θ®sΘ©ΓςH8
A.ΔΌΔήB.ΔήC.ΔΎΔέΔήD.ΔΌΔΎΔέ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΕύ≥ΏΕ»Η¥‘”Μ·―ßœΒΆ≥ΡΘ–ΆΩ…“‘”ΟΝΩΉ”Μ·―ßΦΤΥψ–Γ«χΦδΡΎ(»γ…ζΈοΙΧΒΣ ±ΙΧΒΣΟΗ÷–)ΒΡΜ·―ßΖ¥”ΠΓΘ
(1)ΙΧΒΣΟΗ”–ΧζΒΑΑΉΚΆνβΧζΒΑΑΉΝΫ÷÷Θ§ΥϋΟ«≤ΜΫωΡήΙΜ¥ΏΜ·N2ΜΙ‘≠≥…NH3Θ§ΜΙΡήΫΪΜΖΨ≥ΒΉΈο““»≤¥ΏΜ·ΜΙ‘≠≥…““œ©ΓΘ
ΔΌ““»≤ «__________(ΧνΓΑΖ«ΦΪ–‘Γ±ΜρΓΑΦΪ–‘Γ±)Ζ÷Ή”ΓΘ
ΔΎΧΦΗΚάκΉ”CH3-ΒΡΝΔΧεΙΙ–ΆΈΣ____________ΓΘ
ΔέΗυΨίΒ»ΒγΉ”‘≠άμΘ§NOΘΪΒΡΒγΉ” ΫΈΣ________________ΓΘ
(2)ΖΑΩ…”Ο”ΎΚœ≥…Βγ≥ΊΒγΦΪΘ§“≤Ω…”Ο”Ύ»ΥΙΛΚœ≥…ΕΰΦέΒΡΖΑΙΧΒΣΟΗ(ΫαΙΙ»γΆΦa)ΓΘ
ΔΌV2ΘΪΜυΧ§ ±ΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ____________________________________________ΓΘ
ΔΎΖΑΙΧΒΣΟΗ÷–ΖΑΒΡ≈δΈΜ‘≠Ή””–_____________________________(Χν‘ΣΥΊΖϊΚ≈)ΓΘ
(3)―ΧθΘΑΖ(ΫαΙΙ»γΆΦb)Ω…”Ο”ΎΚœ≥…ΙβΚœΗ®ΟΗNADPHΘ§―ΧθΘΑΖΖ÷Ή”÷–ΒΣ‘≠Ή”ΒΡ‘”Μ·ΙλΒάάύ–Ά”–_______________________Θ§1 molΗΟΖ÷Ή”÷–Κ§Π“ΦϋΒΡ ΐΡΩΈΣ________ΓΘ
(4)12 g ·ΡΪœ©(ΫαΙΙ»γΆΦc)÷–Κ§”–ΒΡ’ΐΝυ±Ώ–Έ ΐΡΩ‘ΦΈΣ________ΘΜ«κΡψ‘Λ≤βΙη «Ζώ»ί“Ή–Έ≥…άύΥΤ ·ΡΪœ©ΒΡΫαΙΙΘ§≤ΔΥΒΟςάμ”…ΘΚ___________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ»γΆΦΈΣΈ“Ιζ‘≤Ος‘Α ό ΉΆ≠œώΓΣΆΟ ΉΚΆ σ ΉΘ§Ω¥…œ»Ξ»‘λΎλΎ…ζΜ‘ΓΘœ¬Ν–Ε‘Τδ‘≠“ρΒΡΖ÷ΈωΉνΩ…ΡήΒΡ «Θ® Θ©

A.ΥϋΟ«±μΟφΒΡΆ≠¬Χ“―”ΟΥα–‘»ή“Κœ¥»ΞB.άϊ”ΟΒγΕΤ‘≠άμ‘ΎΤδ±μΟφΕΤΝΥ“Μ≤ψΡΆΗ· ¥ΒΡΜΤΫπ
C.Ά≠ΒΡΜνΕ·–‘Κή»θΘ§”κΩ’Τχ≥…Ζ÷≤ΜΖ¥”ΠD.ΥϋΟ« «Κ§“ΜΕ®±»άΐΫπΓΔ“χΓΔΈΐΓΔ–ΩΒΡΆ≠ΚœΫπ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΝρΥα…ζ≤ζ÷–¥φ‘Ύ¬·ΤχΉΣΜ·Ζ¥”ΠΘΚ2SO2Θ®gΘ©+O2Θ®gΘ©![]() 2SO3Θ®gΘ©ΓΘ―–ΨΩΖΔœ÷Θ§SO3ΒΡΧεΜΐΖ÷ ΐΥφΈ¬Ε»Θ®TΘ©ΒΡ±δΜ·»γ«ζœΏIΥυ ΨΓΘœ¬Ν–≈–Εœ’ΐ»ΖΒΡ «
2SO3Θ®gΘ©ΓΘ―–ΨΩΖΔœ÷Θ§SO3ΒΡΧεΜΐΖ÷ ΐΥφΈ¬Ε»Θ®TΘ©ΒΡ±δΜ·»γ«ζœΏIΥυ ΨΓΘœ¬Ν–≈–Εœ’ΐ»ΖΒΡ «

AΘ°ΗΟΖ¥”ΠΒΡ’ΐΖ¥”ΠΈΣΈϋ»»Ζ¥”Π
BΘ°Ζ¥”Π¥οΒΫBΒψ ±Θ§2Π‘’ΐΘ®O2Θ©=Π‘ΡφΘ®SO3Θ©
CΘ°«ζœΏI…œAΓΔCΝΫΒψΖ¥”ΠΥΌ¬ ΒΡΙΊœΒΘΚΠ‘A>Π‘C
DΘ°“―÷ΣV2O5ΒΡ¥ΏΜ·–ßΙϊ±»Fe2O3ΚΟΘ§»τI±μ Ψ”ΟV2O5Ής¥ΏΜ·ΦΝ ±ΒΡ«ζœΏΘ§‘ρII «Fe2O3Ής¥ΏΜ·ΦΝ ±ΒΡ«ζœΏ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ‘Ύ500 mL KNO3ΚΆCu(NO3)2ΒΡΜλΚœ»ή“Κ÷–Θ§c(NO3-)ΘΫ6 mol/LΘ§”Ο ·ΡΪΒγΦΪΒγΫβ¥Υ»ή“ΚΘ§Β±Ά®Βγ“ΜΕΈ ±ΦδΚσΘ§ΝΫΦΪΨυ ’Φ·ΒΫ22.4 LΤχΧε(±ξΉΦΉ¥Ωω)Θ§ΦΌΕ®ΒγΫβΚσ»ή“ΚΧεΜΐ»‘ΈΣ500 mLΘ§œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
A. ΒγΫβΒΟΒΫΒΡCuΒΡΈο÷ ΒΡΝΩΈΣ0.5 mol
B. œρΒγΫβΚσΒΡ»ή“Κ÷–Φ”»κ98 gΒΡCu(OH)2Ω…Μ÷Η¥ΈΣ‘≠»ή“Κ
C. ‘≠ΜλΚœ»ή“Κ÷–c(KΘΪ)ΘΫ4 mol/L
D. ΒγΫβΚσ»ή“Κ÷–c(HΘΪ)ΘΫ2 mol/L
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ
Θ®1Θ©œ¬Ν–Νυ÷÷…ζΜν÷–≥ΘΦϊΈο÷ ΘΚ
ΔΌ ≥―Έ ΔΎ ≥”Ο”Ά ΔέλiΟΙΥΊ ΔήΦΠΒΑ ΔίΈςΚλ Ν ΔόΥήΝœ
Α¥“Σ«σ”ΟΈο÷ –ρΚ≈ΧνΩ’ΘΚ
ΉςΒςΈΕΦΝΒΡ «___________ΘΜΉςΩΙ…ζΥΊΒΡ «___________ΘΜΗΜΚ§ΒΑΑΉ÷ ΒΡ «___________ΘΜΗΜΚ§Έ§…ζΥΊΒΡ «___________ΘΜΗΜΚ§”Ά÷§ΒΡ «___________ΘΜ τ”ΎΚœ≥…≤ΡΝœΒΡ «___________ΓΘ
Θ®2Θ©“ΜΗωΧε÷Ί50 kgΒΡΫΓΩΒ»ΥΘ§ΧεΡΎ‘ΦΚ§”–2 gΧζΘ§’β2 gΧζ‘Ύ»ΥΧεΡΎ≤Μ «“‘ΒΞ÷ ΒΡ–Έ Ϋ¥φ‘ΎΘ§Εχ «“‘Fe2+ΚΆFe3+ΒΡ–Έ Ϋ¥φ‘ΎΓΘ’ΐΕΰΦέΧζάκΉ”“Ή±ΜΈϋ ’Θ§ΗχΤΕ―Σ’Ώ≤Ι≥δΧζ ±Θ§”ΠΗχ”ηΚ§Fe2+ΒΡ―«Χζ―ΈΘ§»γΝρΥα―«ΧζΓΘΖΰ”ΟΈ§…ζΥΊCΘ§Ω… Ι ≥Έο÷–ΒΡFe3+ΜΙ‘≠≥…Fe2+Θ§ ”–άϊ”Ύ»ΥΧεΈϋ ’ΓΘ
i.‘Ύ»ΥΧε÷–Ϋχ––Fe2+ ![]() Fe3+ΒΡΉΣΜ· ±Θ§ΔΌ÷–ΒΡFe2+Ής________ (ΧνΓΑ―θΜ·ΦΝΓ±ΜρΓΑΜΙ‘≠ΦΝΓ±)Θ§ΔΎ÷–ΒΡFe3+Ής________ (ΧνΓΑ―θΜ·ΦΝΓ±ΜρΓΑΜΙ‘≠ΦΝΓ±)ΓΘ
Fe3+ΒΡΉΣΜ· ±Θ§ΔΌ÷–ΒΡFe2+Ής________ (ΧνΓΑ―θΜ·ΦΝΓ±ΜρΓΑΜΙ‘≠ΦΝΓ±)Θ§ΔΎ÷–ΒΡFe3+Ής________ (ΧνΓΑ―θΜ·ΦΝΓ±ΜρΓΑΜΙ‘≠ΦΝΓ±)ΓΘ
ii.Ζΰ”ΟΈ§…ζΥςCΘ§Ω… Ι ≥Έο÷–ΒΡFe3+ΜΙ‘≠≥…Fe2+’βΨδΜΑ÷Η≥ω,Έ§…ζΥΊC‘Ύ’β“ΜΖ¥”Π÷–Ής ____________Θ®ΧνΓΑ―θΜ·ΦΝΓ±ΜρΓΑΜΙ‘≠ΦΝΓ±Θ©
iii. –≥Γ≥ω έΒΡΡ≥÷÷¬σΤ§÷–Κ§”–ΈΔΝΩΒΡΩ≈ΝΘœΗ–ΓΒΡΜΙ‘≠ΧζΖέΘ§’β–©ΧζΖέ‘Ύ»ΥΧεΈΗΥα (HC1)ΒΡΉς”Οœ¬ΉΣΜ·≥…―«Χζ―ΈΓΘ¥ΥΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣ___________________________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΫ®‘λΫΔ¥§–η“Σ¥σΝΩΒΡ–¬–Ά≤ΡΝœΘ§ΫΔ¥§ΒΡΦΉΑε“≤“ΣΡΆΗΏΈ¬«“ΆβΩ«“ΣΡΆΗ· ¥Θ§ΕχΡχΗθΧζΚœΫπΨΆ «ΨΏ”–ΗΏ«ΩΕ»ΓΔΡΆΗΏΈ¬ΓΔΡΆΗ· ¥Β»”≈ΝΦ–‘ΡήΒΡΧΊ÷÷Η÷Θ§’βάύΧΊ÷÷Η÷÷–Κ§”–ΧΦΓΔΙηΓΔ―θΓΔΒΣΓΔΝΉΒ»‘ΣΥΊΓΘ
Θ®1Θ©±ΘΗθΗ÷ΩΙΗ· ¥–‘Ρή«ΩΘ§![]() ΜυΧ§‘≠Ή”ΒΡΦέΒγΉ”≈≈≤Φ_________Θ§ΈΣΗθ‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–_________«χΓΘ
ΜυΧ§‘≠Ή”ΒΡΦέΒγΉ”≈≈≤Φ_________Θ§ΈΣΗθ‘ΣΥΊ‘Ύ÷ήΤΎ±μ÷–_________«χΓΘ
Θ®2Θ©![]() Ρή–Έ≥…Εύ÷÷≈δάκΉ”Θ§»γ
Ρή–Έ≥…Εύ÷÷≈δάκΉ”Θ§»γ![]() ΓΔ
ΓΔ![]() ΚΆ
ΚΆ![]() Β»Θ§
Β»Θ§![]() ÷––Ρ‘≠Ή”ΒΡ≈δΈΜ ΐ «_________Θ§”κ
÷––Ρ‘≠Ή”ΒΡ≈δΈΜ ΐ «_________Θ§”κ![]() ΜΞΈΣΒ»ΒγΉ”ΧεΒΡΖ÷Ή”ΈΣ_________ΓΘ
ΜΞΈΣΒ»ΒγΉ”ΧεΒΡΖ÷Ή”ΈΣ_________ΓΘ
Θ®3Θ©ΫΔ¥§ΦΉΑεΆΩ”–“Μ≤ψΡΆΗΏΈ¬ΒΡ≤ΡΝœΨέΙη―θΆιΫαΙΙ»γΆΦΥυ ΨΘ§Τδ÷–![]() ‘≠Ή”‘”Μ·ΖΫ ΫΈΣ________‘”Μ·ΓΘ
‘≠Ή”‘”Μ·ΖΫ ΫΈΣ________‘”Μ·ΓΘ
Θ®4Θ©ΧΦΚΆΙηΩ…Μ·Κœ≥…ΧΦΜ·ΙηΘ§![]() ΨßΧεΨΏ”–άύΥΤΫπΗ’ ·ΒΡΫαΙΙΘ§Τδ÷–ΧΦ‘≠Ή”ΚΆΙη‘≠Ή”ΒΡΈΜ÷Ο «ΫΜΧφΒΡΘ§ΒΪ «ΧΦΜ·ΙηΒΡ»έΒψΒΆ”ΎΫπΗ’ ·Θ§‘≠“ρ «_________ΓΘ
ΨßΧεΨΏ”–άύΥΤΫπΗ’ ·ΒΡΫαΙΙΘ§Τδ÷–ΧΦ‘≠Ή”ΚΆΙη‘≠Ή”ΒΡΈΜ÷Ο «ΫΜΧφΒΡΘ§ΒΪ «ΧΦΜ·ΙηΒΡ»έΒψΒΆ”ΎΫπΗ’ ·Θ§‘≠“ρ «_________ΓΘ
Θ®5Θ©ΧζΒΡ―θΜ·Έο÷°“Μ «![]() Θ§¥”
Θ§¥”![]() ÷–»Γ≥ωΒΡΡήΧεœ÷ΤδΨßΧεΫαΙΙΒΡ“ΜΗωΝΔΖΫΧεΘ§‘ρΨßΧε÷–ΒΡάκΉ” «ΖώΙΙ≥…ΝΥΟφ–ΡΝΔΖΫΉνΟήΕ―ΜΐΘΩ________Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΘΜΗΟΝΔΖΫΧε «≤Μ «
÷–»Γ≥ωΒΡΡήΧεœ÷ΤδΨßΧεΫαΙΙΒΡ“ΜΗωΝΔΖΫΧεΘ§‘ρΨßΧε÷–ΒΡάκΉ” «ΖώΙΙ≥…ΝΥΟφ–ΡΝΔΖΫΉνΟήΕ―ΜΐΘΩ________Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΘΜΗΟΝΔΖΫΧε «≤Μ «![]() ΒΡΨßΑϊΘΩ_________Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΘΜΝΔΖΫΧε÷–ΧζάκΉ”¥Π”Ύ―θάκΉ”Έß≥…ΒΡ_________Θ®ΧνΩ’ΦδΫαΙΙΘ©Ω’œΕΘΜΗυΨί…œΆΦΦΤΥψ
ΒΡΨßΑϊΘΩ_________Θ®ΧνΓΑ «Γ±ΜρΓΑΖώΓ±Θ©ΘΜΝΔΖΫΧε÷–ΧζάκΉ”¥Π”Ύ―θάκΉ”Έß≥…ΒΡ_________Θ®ΧνΩ’ΦδΫαΙΙΘ©Ω’œΕΘΜΗυΨί…œΆΦΦΤΥψ![]() ΨßΧεΒΡΟήΕ»ΈΣ_________
ΨßΧεΒΡΟήΕ»ΈΣ_________![]() ΓΘΘ®ΆΦ
ΓΘΘ®ΆΦ![]() Θ§ΦΤΥψΫαΙϊ±ΘΝτΝΫΈΜ”––ß ΐΉ÷Θ©
Θ§ΦΤΥψΫαΙϊ±ΘΝτΝΫΈΜ”––ß ΐΉ÷Θ©
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩΡ≥ΕΧ÷ήΤΎΖ«Ϋπ τ‘ΣΥΊΒΡ‘≠Ή”ΚΥΆβΉνΆβ≤ψΒγΉ” ΐ «¥ΈΆβ≤ψΒγΉ” ΐΒΡ“ΜΑκΘ§ΗΟ‘ΣΥΊ
A. ‘ΎΉ‘»ΜΫγ÷–÷Μ“‘Μ·ΚœΧ§ΒΡ–Έ Ϋ¥φ‘Ύ
B. ΒΞ÷ ≥Θ”ΟΉςΉσΑκΒΦΧε≤ΡΝœΚΆΙβΒΦœΥΈ§
C. ΉνΗΏΦέ―θΜ·Έο≤Μ”κΥαΖ¥”Π
D. ΤχΧ§«βΜ·Έο±»ΦΉΆιΈ»Ε®
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com