
��λ����һ������Ĺ��ۼ��������õ��Ӷ���ijԭ�ӵ������ṩ����һȱ���ӵ����ӽ�ϡ���NH4��������NH3����ԭ���ṩ���Ӷԣ���H����ȱ���ӣ�ͨ����λ���γɵġ��ݴˣ��ش��������⣺
��1�����������п��ܴ�����λ������________��
| A��CO2 | B��H3O�� | C��CH4 | D��H2SO4 |
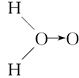 ���ң�H��O��O��H��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѡ���ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺a.��C2H5OH��ŨH2SO4��Ӧ���ɣ�C2H5��2SO4��ˮ��b.���Ƶõģ�C2H5��2SO4��H2O2��Ӧ��ֻ����A��H2SO4��c.�����ɵ�A��H2��Ӧ����֪�÷�Ӧ��H2����ԭ������
���ң�H��O��O��H��ʽ��O��O��ʾ��λ�����ڻ�ѧ��Ӧ��O��O��������ԭ��ʱ���ѡ���ѧ��Baeyer��VilliyerΪ�о�H2O2�Ľṹ����Ʋ����������ʵ�飺a.��C2H5OH��ŨH2SO4��Ӧ���ɣ�C2H5��2SO4��ˮ��b.���Ƶõģ�C2H5��2SO4��H2O2��Ӧ��ֻ����A��H2SO4��c.�����ɵ�A��H2��Ӧ����֪�÷�Ӧ��H2����ԭ������ ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ��ش��������⣺
��1���������������ʣ���̼60��C60������������Na2O����� CaF2�����P4O10�����̼���辧�塣�����������Ӿ������ �����ڷ��Ӿ������ ������ԭ�Ӿ������____��
��2�������з��ӣ�HCN��P4��SO3��PCl3��BF3���������ڷǼ��Է��ӵ��� ��
��3�����������ӣ�SO32-��SO42-��CO32-������VSEPRģ��Ϊ���������ε��� ������ԭ�ӵ��ӻ������������sp2�ӻ����� ��
��4��CaO��������ͼ��ʾ��CaO������Ca2������λ��Ϊ______����ÿһ�������Ӿ���������Ҿ�����ȵĸ�������____�� ��CaO�����NaCl����ľ����ֱܷ�Ϊ��CaO��3 401 kJ/mol��NaCl��786 kJ/mol���������߾����ܲ������Ҫԭ��_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о���Ա������֣���һ����ʵ�������£���ˮʩ��һ�����糡����20�桢1������ѹ�£�ˮ���Խ�ɱ�����Ϊ���ȱ�����ͼ����ˮ�͡��ȱ����ļ����ģ��ͼ��ͼ�������ˮ�����е�ԭ�ӡ�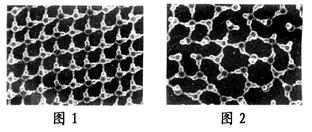
ͼ��
��1��ͼ�нϴ�������________ԭ�ӣ���ԭ�ӽṹʾ��ͼ��________��ˮ����������ԭ�Ӽ�Ļ�ѧ����________������ۼ��������Ӽ�������
��2�������ģ�ͱ�ʾ��ˮ���ӽṹ��________��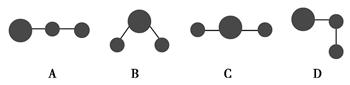
ͼ��
��3����֪ˮ��������ԭ��һ�˴����ָ���ɣ���ԭ��һ�˴���������ɣ�����ӵ糡�����£�ˮ��ɱ���ͼ����ģ�⡰�ȱ�����ʾ��ͼ��________���ͼ1����ͼ2������������_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������γɶ������ӣ���N3����NH2����N3����NH4+��N2H5+��N2H62+�ȣ���֪N2H5+
��N2H62+�������Է��ӽ�������γɵģ�������NH4+������������� NH4+�����ʡ�
��д��N2H62+�ڼ�����Һ�з�Ӧ�����ӷ���ʽ ��
��NH2���ĵ���ʽΪ ��
��N3���� �����ӡ�
��д�������ɶ��ԭ����ɵĺ�����N3����������ͬ�����ʵĻ�ѧʽ �� ��
�ɵȵ��������������������ƵĽṹ����Ԥ��N3���Ĺ��� ��
�ʾݱ�����������ѧ�ҿ���������˹����1998��11�ºϳ���һ����Ϊ��N5�������ʣ���������м�ǿ�ı�ը�ԣ��ֳ�Ϊ������ը����������Ϊֹ�����Ƕ����Ľṹ�в������ֻ֪����N5��ʵ�����Ǵ�����ɵķ�����Ƭ����ṹ�ǶԳƵģ�5��N�ų�V�Ρ����5��N��Ϻﵽ8���ӽṹ���Һ���2��N��N������N5��������Ƭ��������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
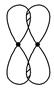
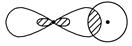
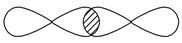
��.��1��д�����е����ƵĽ���ͼ��ʾ���γɵļ������ͣ�
��
_________________________________________________________________��
��
_________________________________________________________________��
��
_________________________________________________________________��
��
_________________________________________________________________��
��2�����з����У�û�й�ѧ���Ե���____________��������������̼ԭ�ӵ���
____________��
| A������CH3��CHOH��COOH |
| B������CH2OH��CHOH��CH2OH |
| C����������CH2OH��CHOH��CHOH��CH2��CHO |
| D������CH2OH��CHOH��CHOH��CHOH��CHO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�õ���ʽ��ʾ�������ʵĽṹ��
(1)NH4Cl____________��(2)Na2O________________��(3)Na2O2________��(4)H2S________��(5)CO2________��(6)MgCl2________��
(1)��(6)�У��������Ӽ�����________���������ۼ�����________���������Ӽ����й��ۼ�����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��H��C��O��Cl��Na����Ԫ�ء�
��1����������Ԫ����ɵĻ�������������ӻ�������(д2��)______________________�����ۻ�������(д2��)______________________��
��2����������Ԫ����ɵĻ�������������ӻ�������(д2��)______________________�����ۻ�������(д2��)______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и������ʵľ����У���ѧ��������ͬ����������Ҳ��ͬ����
| A��SO2��Si | B��CO2��H2O |
| C��NaCl��HCl | D��CCl4��KCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й����Ӽ����������˵����ȷ����
| A��HCI����ˮʱ��H�� Cl������� |
| B��H2O2������ֻ�м��Լ� |
| C��NH3���ԷǼ��Լ���ϵķ��� |
| D��MgCl2�м������Ӽ������й��ۼ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com