
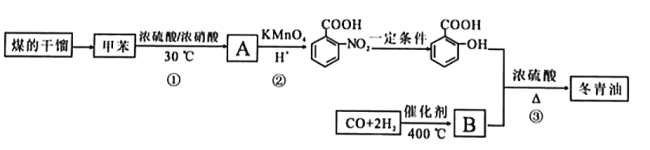
����Ŀ���ױ���ú����IJ���,�������Ʊ�����ֹʹ����Ч���Ķ�����( 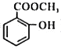 )���ϳ�·�����£�
)���ϳ�·�����£�
��֪��
��ش���������
��1��ú�ĸ�����_______________��(���������仯��������ѧ�仯��).
��2��A�Ľṹ��ʽΪ_______________����Ӧ�ٵķ�Ӧ������_______________��
��3����Ӧ�ڵķ�Ӧ������_______________��
��4��B�Ĺ���������Ϊ_______________��
��5����Ӧ�۵Ļ�ѧ����ʽ_______________��
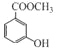
��6��C�Ƕ����͵�ͬ���칹�壬�䱽���ϵ�ȡ�����붬������ͬ����C�Ľṹ��ʽ����Ϊ__________��
���𰸡���ѧ�仯 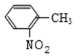 ȡ����Ӧ ������Ӧ �ǻ�
ȡ����Ӧ ������Ӧ �ǻ� ![]() +CH3OH
+CH3OH![]()
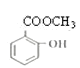 +H2O ��
+H2O ��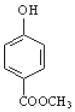 ��
�� ��
��
��������
�ױ���Ũ���ᷢ��ȡ����Ӧ����A��A����������Ӧ���������������ᣬ����������������֪���ױ������ᷢ����λȡ������AΪ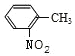 ����������������һ�������·�Ӧ�������ǻ������ᣬB�����ǻ������ᷴӦ����
����������������һ�������·�Ӧ�������ǻ������ᣬB�����ǻ������ᷴӦ���� ����BΪCH3OH�������Ŀ�������
����BΪCH3OH�������Ŀ�������
(1)ú�ĸ�������������������ɣ����Ը����ǻ�ѧ�仯��
�ʴ�Ϊ����ѧ�仯��
(2)A�Ľṹ��ʽΪ ����Ӧ�ٵķ�Ӧ������ȡ����Ӧ��
����Ӧ�ٵķ�Ӧ������ȡ����Ӧ��
�ʴ�Ϊ�� ��ȡ����Ӧ��
��ȡ����Ӧ��
(3)��Ӧ�ڵķ�Ӧ������������Ӧ��
�ʴ�Ϊ��������Ӧ��
(4)BΪCH3OH��B�Ĺ���������Ϊ�ǻ���
�ʴ�Ϊ���ǻ���
(5)��Ӧ�۵Ļ�ѧ����ʽΪ![]() +CH3OH
+CH3OH![]()
 +H2O��
+H2O��
�ʴ�Ϊ��![]() +CH3OH
+CH3OH![]()
 +H2O��
+H2O��
(6)������Ϊ ��C�Ƕ����͵�ͬ���칹�壬�䱽���ϵ�ȡ�����붬������ͬ��ΪCOOCH3��OH����C�Ľṹ��ʽ����Ϊ
��C�Ƕ����͵�ͬ���칹�壬�䱽���ϵ�ȡ�����붬������ͬ��ΪCOOCH3��OH����C�Ľṹ��ʽ����Ϊ ��
�� ��
��
�ʴ�Ϊ�� ��
�� ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�Ͽ���ˮú���ϳɼ״���
��1��������ͨ�����з�Ӧ�Ʊ��״���CO(g)+2H2(g)![]() CH3OH(g)����ͼ�Ƿ�ӦʱCO(g)��CH3OH(g)��Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��CO��ʾƽ����Ӧ����v(CO)=__________���÷�Ӧ��ƽ�ⳣ������ʽΪ__��
CH3OH(g)����ͼ�Ƿ�ӦʱCO(g)��CH3OH(g)��Ũ����ʱ��t�ı仯������ӷ�Ӧ��ʼ��ƽ�⣬��CO��ʾƽ����Ӧ����v(CO)=__________���÷�Ӧ��ƽ�ⳣ������ʽΪ__��

��2����һ�ݻ��ɱ���ܱ������г���10mol CO��20mol H2��CO��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ��
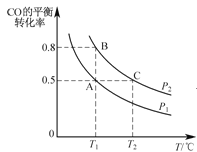
������˵�������жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����_________��������ĸ��
A��H2���������ʵ���CH3OH���������ʵ�2��
B��H2������������ٸı�
C����ϵ��H2��ת���ʺ�CO��ת�������
D����ϵ�������ƽ��Ħ���������ٸı�
�ڱȽ�A��B����ѹǿ��СPA_PB��������������=������
�����ﵽ��ѧƽ��״̬Aʱ�����������Ϊ20 L�������Ӧ��ʼʱ�Գ���10 mol CO��20 mol H2������ƽ��״̬Bʱ���������V(B)=__L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������غ����У������뺣���й�˵����ȷ����( )
A.�Ӻ�ˮ����ȡ![]() ���ʣ����һ�����
���ʣ����һ�����![]() ������Һ���Ϳ��Ƶý���þ
������Һ���Ϳ��Ƶý���þ
B.��ˮ����һ���������̿��������ɱ����84����Һ
C.�Ӻ�������ȡ�⣬��![]() ����Һ�����þƾ���ȡ
����Һ�����þƾ���ȡ
D.��ˮ�и�����Ԫ������Ŀǰ��֪��Ԫ���е縺����ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪���ٱ�״���£�1���ˮ��������ܽ�500�����HCl���ڱ���NaCl��Һ��Ũ��ԼΪ5.00mol��L��1��
��1���ڱ�״���£���448LHCl��������1 Lˮ�У�������ҺA���ܶ�Ϊ1.20 g��cm-3������ҺA��HCl�����ʵ���Ũ��Ϊ____��(�����������ȡ��λ��Ч����)
��2����ʹ1L�����Ȼ�����Һ��Cl��Ũ������ҺA�е�Cl��Ũ����ȣ�����1LNaCl������Һ�л�Ӧ�ܽ�Լ___L��״����HCl����(��Һ����仯���Բ��ƣ��������Ȼ�������)��
��3������10.0mL��ҺAϡ�ͳ�500mL��ҺB������ҺB��HCl�����ʵ���Ũ��Ϊ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ش�����ʵ��������ʵ��������й����⡣


(1)ͼA���Թܼе�������______________________��
(2)ָ��ͼB�еĴ��������____________________________________��
(3)ͼC��D��ijͬѧ����50 g��������Ϊ6%���Ȼ�����Һʱ���ڳ�������ȡ�������������г��ֵ������
����ͼC��ʾ���ڳ����Ȼ��ƵIJ��������У�����ָ��ƫ�ң���������еIJ�����______________������ƽƽ�⡣
����ͼD��ʾ����ȡˮʱ�����Ӷ�����������Һ�����ʵ�����������______________(����ƫ������ƫС������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��оƬ��Ҫ�ɵ�����������ͼ�ǹ輰�仯�������۶�άͼ������Ҫ��������л�ѧ����ʽ�����ӷ���ʽ��
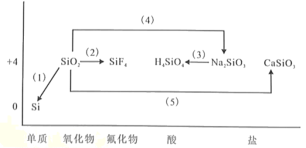
��1�����ý�̿�ڵ�¯�л�ԭ��������Ļ�ѧ����ʽΪ_______________��
��2������������ʴ�����Ļ�ѧ����ʽΪ_______________��
��3��������ת��Ϊԭ��������ӷ���ʽ_______________��
��4�������������ռ���Һ��Ӧ�Ļ�ѧ����ʽ_______________��
��5��ʯӢ�봿���ڲ�����¯���ǿ�ȷ�����Ӧ�Ļ�ѧ����ʽΪ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ӦA+B��C ��H ��0������������ �� A+B��X ��H��0 �� X��C ��H��0 ������ʾ��ͼ�У�����ȷ��ʾ�ܷ�Ӧ�����������仯����
A.  B.
B. 
C.  D.
D. 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ȤС���Ʊ�һ��������������.ȡ3mL��ˮ�Ҵ�,2mLŨ����,2mL���������ʵ�飬��5mL����̼������Һ�ռ����
I.ʵ��װ����ͼ��ʾ
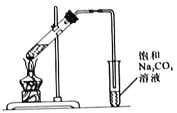
��1���Ʊ����������Ļ�ѧ����ʽΪ_______________��
��2��Ũ�����������_______________��
��3�������ܵ�������_______________��
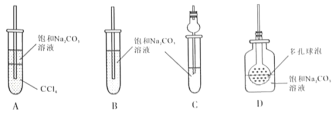
��4������װ�û���ѡ����ͼ�е�___________��(�����).
��.��ͬѧ�ú��з�̪�ı���̼������Һ(�ʼ���)�ռ�����������ֺ�ɫѸ����ȥ.
��ͬѧ��Ϊ�������������к���̼����.��ͬѧͨ���������ϲ���������ʵ�飬֤����ͬѧ���Ʋ��Ǵ���ġ�
��֪����̪������ˮ���������л��ܼ�����̪�Լ��Ƿ�̪���Ҵ���Һ.
ʵ��i��ȡ����²���ɫҺ�壬�ֳ����ݣ��ֱ��������ʵ��
��� | ʵ����� | ʵ������ | ���� |
1 | �μӼ�����̪�Լ� | ��Һ �� (��������������������) | ̼���Ʋ�δ��������ȫ�кͣ����д���ʣ�� |
2 | ����������Һ | �д������ݲ��� |
ʵ��ii.ȡ����ϲ�Һ�壬���� �� ��Һ�������ֳ���dz��ɫ�����÷ֲ���ɫ��ʧ��
ʵ��iii��ȡ5mL����̼������Һ�����뼸�η�̪�Լ����ټ���3mL��������(��������)����Һ�ȱ�죬���ɫ��ʧ���ش���������
��5���������ʵ�飺��_______________����_______________��
��6�����ʵ��ii��ʵ��iii�����ɵó��Ľ�����_______________��
��7��ʵ��iii��ʵ��Ŀ����_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£���10 L�ܱ������м���5 mol SO2��3 mol O2��������Ӧ��2SO2(g)��O2(g)![]() 2SO3(g)��10 minʱ����Ӧ�ﵽƽ��״̬����ʱ��3 mol SO2�����˷�Ӧ��
2SO3(g)��10 minʱ����Ӧ�ﵽƽ��״̬����ʱ��3 mol SO2�����˷�Ӧ��
��1����Ӧ������________ mol SO3��v(SO2)��________��
��2��ƽ��ʱSO3��Ũ����________��SO2��ת������________��
��3��ƽ��ʱ����������������ʵ���Ϊ________mol��
��4�����ʵ�Ũ�Ȳ��ٸı��־�Ÿ÷�Ӧ�Ѵ�ƽ�⣬���л�����˵���÷�Ӧ�Ѵ�ƽ�����______(�����)��
����ϵ��ѹǿ���ٸı� ��������������ܶȲ��ٸı�
�ۻ�������ƽ����Է����������ٸı�
��v��(SO3)��2v��(O2) ��n(SO3)��n(O2)��n(SO2)��2��1��2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com