
����Ŀ�����о����������ֵĹ����У�ͨ������������Cu��Ni��Zn��Sn��Fe����������
(1)ij�ֽ�������������Է��ԣ�ԭ������ά�ռ�����������������У��ý�������������________(���������������Ǿ�����)
(2)��̬Ni2���ĺ�������Ų�ʽΪ________��Ni2����Fe2���İ뾶�ֱ�Ϊ69 pm��78 pm�����۵�NiO______FeO(����<������>��)����ԭ����_________________________________________��
(3)ͭ������±��(SCN)2��Ӧ����Cu(SCN)2��1 mol (SCN)2�����к�����������ĿΪ________��д��һ����SCN����Ϊ�ȵ�����ķ���________(�û�ѧʽ��ʾ)��
(4)��������ͭ�ķ��ӽṹ��ͼ��̼ԭ�ӵ��ӻ���ʽΪ________��

(5)����NiO(������)����Ľṹ��ͼ��ʾ���侧���߳�Ϊa pm����ʽ��ʾNiO������ܶ�Ϊ________g��cm��3(���ؼ��������������ӵ�������ֵΪNA)���˹��Ʊ���NiO�����г�����ȱ��(��ͼ)��һ��Ni2����ȱ����������Ni2��������Ni3����ȡ�������������Գʵ����ԣ�����������Ni��O�ı�ֵȴ�����˱仯����֪ij��������Ʒ���Ni0.96O���þ�����Ni3����Ni2�������Ӹ���֮��Ϊ________��

���𰸡����� 1s22s22p63s23p63d8��[Ar]3d8 > Ni2+��Fe2+���������������ͬ��Ni2+���Ӱ뾶��С��NiO�����Ӽ���ǿ��NiO������۵���� 5NA(��5��6.02��1023��3.01��1024) CO2 sp3��sp2 ![]() 1��11
1��11
��������
��1��ij�ֽ�������������Է��ԣ�ԭ������ά�ռ�����������������У��ý������������ھ��壻
�ʴ�Ϊ�����壻
��2��NiԪ��ԭ�Ӻ��������Ϊ28����������Ų�ʽΪ1s22s22p63s23p63d84s2��ʧȥ4s�ܼ�2�������γ�Ni2+����Ni2+���Ӻ�������Ų�Ϊ��1s22s22p63s23p63d8��
Ni2+��Fe2+���������������ͬ��Ni2+���Ӱ뾶��С��NiO�����Ӽ���ǿ��NiO������۵���ߣ����۵�NiO>FeO��
�ʴ�Ϊ��1s22s22p63s23p63d8��>��Ni2+��Fe2+���������������ͬ��Ni2+���Ӱ뾶��С��NiO�����Ӽ���ǿ��NiO������۵���ߣ�
��3��(SCN)2�ĽṹʽΪN��C-S-S-C��N������[(SCN)2]�Ľṹ��֪��������3��������2��̼������������ȫΪ��������������1��������2��������1����SCN��2���Ӻ���5����������1mol(SCN)2�����к�����������ĿΪ 5NA��
һ����SCN-��Ϊ�ȵ������ԭ��������ͬ���۵���������Ϊ16�����ϵķ�����CO2�ȣ�
�ʴ�Ϊ��5NA��CO2��
��4�����������Ӱ�����Cԭ���γ�2��C-H����1��C-N��1��C-C����û�йµ��Ӷԣ��ӻ������ĿΪ4����ȡsp3�ӻ�����̼��˫���е�Cԭ���γ�3��������û�йµ��Ӷԣ��ӻ������ĿΪ3����ȡsp2�ӻ���
�ʴ�Ϊ��sp3��sp2��
(5)������Ni2+��ĿΪ1+12��![]() =4��O2-��ĿΪ8��
=4��O2-��ĿΪ8��![]() +6��
+6��![]() =4����������Ϊ4��
=4����������Ϊ4��![]() g�������߳�Ϊapm���������Ϊ��a��10-10 cm��3��NiO������ܶ�ΪΪ4��
g�������߳�Ϊapm���������Ϊ��a��10-10 cm��3��NiO������ܶ�ΪΪ4��![]() g����a��10-10 cm��3=
g����a��10-10 cm��3=![]() g/cm3��
g/cm3��
��1mol Ni0.96O�к�Ni3+xmol��Ni2+Ϊ��0.96-x��mol�����ݾ����Գʵ����ԣ���֪ 3x+2����0.96-x��=2��1��x=0.08mol ��Ni2+Ϊ��0.96-x��mol=0.88mol����������֮��ΪNi3+��Ni2+=0.08��0.88=1��11��
�ʴ�Ϊ��![]() ��1��11��
��1��11��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ���������(��������δ���)��
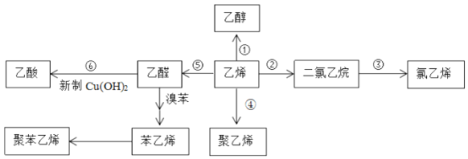
(1)�Ҵ��������ᷴӦ�����й���ζ�����ʣ�������Ϊ____________��
(2)д����Ӧ���ͣ���_____________����______________��
(3)��Ӧ�Ļ�ѧ����ʽ��____________________________________��ʵ��������______________________________��
(4)��Ӧ����KOH���Ҵ���Һ������������������ϩ�ķ���ʽΪ��____________________________________________________________________��
(5)������ϩ�����������ŵ�����_____________________________________��
(6)����ϩ�ϳɾ۱���ϩ�Ļ�ѧ����ʽ��__________________________________________��
(7)���Ҵ��ͱ���ϩΪԭ�Ϻϳ��л���![]() ��д���ϳ�·��ͼ_____________________________��(�ϳ�·�߳��õı�ʾ��ʽΪ��A
��д���ϳ�·��ͼ_____________________________��(�ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B
B![]()
![]() Ŀ�����)
Ŀ�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȩ��HCHO���ڻ�����ҽҩ��ũҩ�ȷ����й㷺��Ӧ�á�
�����ü״���CH3HO���Ʊ���ȩ
���ⷨ��CH3OH��g�� HCHO��g��+H2 (g) ��H1=+92.09kJ��mol -1
��������CH3OH��g��+1/2O2��g��HCHO��g��+H2O��g�� ��H2
��1�����ⷨ�Ƽ�ȩ�����������ƽ����ʵ�������________ ��д��һ������
��2����֪��2 H2��g�� + O2��g��= 2H2O��g�� ��H3=-483.64kJ��mol -1������H2=________________��
��3��750K �£��ں����ܱ������У�����һ�����ļ״����������ⷨ��Ӧ������ʼѹǿΪP0���ﵽƽ��ʱת����Ϊ40.0% ����Ӧ��ƽ�ⳣ��Kp=________����ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������������������Ӧ����
��4��Na2CO3�����ⷨ��Ӧ�Ĵ��������о�ָ��������Ӧ�IJ��ֻ������£�
����i ��CH3OH����H+ ��CH2OH ����ii ����CH2OH����H+ HCHO
����iii������CH2OH��3��H +CO ����iv����H+��H��H2
��ͼ��ʾΪ�����Ϊ1L�ĺ��������У�Ͷ��1mol CH3OH����̼���ƴ��������£�����10min��Ӧ����ü״���ת���ʣ�X����״���ѡ���ԣ�S�����¶ȵĹ�ϵ����ȩ��ѡ���ԣ�ת����CH3OH������HCHO�İٷֱȣ����ش��������⣺
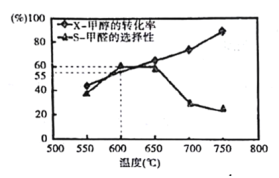
��600��ʱ��ǰ10min�ڼ�ȩ��ƽ������Ϊv��HCHO��=______
�ڴ�ƽ��Ƕȷ���550��- 650��״����ɼ�ȩ��ת�������¶����ߵ�ԭ��Ϊ______��
�۷�Ӧ����i�Ļ��______������>����<�� ����=����CH3OH��g��HCHO��g��+H2��g����ܡ�
��650��- 750�淴Ӧ����ii������_______������>����<�� ����=������Ӧ����iii�����ʡ�
��ȩ�����Σ�����彡������Լ�ȩ������⼰������
ij��ȩ����̽��������ȼ�ϵ�ع���ԭ����b�缫��Ӧ����ʽΪ________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͭ����Ͻ�����������ʹ�õĽ������ϡ�
��1����ͼ�ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ___________��

��2��Cu2������NH3��H2O��Cl�����γ���λ��Ϊ4������
�٣�Cu(NH3)4��2���д��ڵĻ�ѧ��������_____________������ţ���
A����λ�� B�������� C�����Թ��ۼ� D���Ǽ��Թ��ۼ� E�����Ӽ�
�ڣ�Cu(NH3)4��2�����жԳƵĿռ乹�ͣ���Cu(NH3)4��2���е�����NH3������Cl��ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ�����Cu(NH3)4��2���Ŀռ乹��Ϊ___________��
��3���������ڹ���Ԫ��Fe��Ti����C��H��N��O�γɶ��ֻ����
��H��C��N��O����Ԫ�صĵ縺����С�����˳��Ϊ_______________________��
��������������ȷ����____________��������ĸ��
A����ΪHCHO��ˮ���Ӽ����γ����������CH2O������ˮ
B��HCHO��CO2�����е�����ԭ�Ӿ�����sp2�ӻ�
C��C6H6�������6��![]() ����1����
����1����![]() ����C2H2�ǷǼ��Է���
����C2H2�ǷǼ��Է���
D��CO2������۵㡢�е㶼�ȶ������辧��ĵ�
�����ᣨHOCN����һ����״���ӣ����������ᣨHNCO����Ϊͬ���칹�壬������ڸ�ԭ���������Ѵﵽ�ȶ��ṹ����д������Ľṹʽ________________��
��4��Feԭ�ӻ�������Χ�н϶���������Ŀչ������һЩ���ӻ������γ�������Feԭ�ӻ������γ������ķ��ӻ�����Ӧ�߱��Ľṹ������_________ д��һ���� CN- ��Ϊ�ȵ�����ĵ��ʷ���ʽ_______________________��
��5��һ��Al��Fe�Ͻ�����徧������ͼ��ʾ����ݴ˻ش��������⣺

��ȷ���úϽ�Ļ�ѧʽ______________��
����������ܶȣ��� g/cm3����˺Ͻ������������Feԭ��֮��ľ���(�ú��ѵĴ���ʽ��ʾ�����ػ���)Ϊ__________cm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��A��B��Cԭ���������ε��������ǵ�ԭ�ӵ�����������֮��Ϊ10��A��C�����ڱ���ͬ���壬Bԭ����������������Aԭ�Ӵ���������������������ȷ����(����)
A. ԭ�Ӱ뾶A��B��C
B. A���⻯����ȶ���С��C���⻯����ȶ���
C. C����������۵��A��������ĵ�
D. A��C���γ�ԭ�Ӿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E����ǰ������ԭ�������������������Ԫ�ء�A��Dͬ�����������ֳ���������DA2��DA3����ҵ�ϵ������C2A3��ȡ����C��B��E��������ֻ��2�������⣬�������ȫ������Eλ��Ԫ�����ڱ���ds�����ش��������⣺
(1)B��C�е�һ�����ܽϴ����________����̬Dԭ�Ӽ۵��ӵĹ������ʽΪ________________��
(2)DA2���ӵ�VSEPRģ����____________��
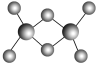
(3)ʵ����C����Ԫ���γɵĻ������ʵ�����ΪC2Cl6�������ģ����ͼ��ʾ����֪C2Cl6�ڼ���ʱ���������������NaOH��Һ��Ӧ������Na[C(OH)4]��
��C2Cl6����________(�������)���壬����Cԭ�ӵ��ӻ��������Ϊ________�ӻ���
��[C(OH)4]���д��ڵĻ�ѧ����____________________________________________��
(4)����A������ͬ�������壬���зе�ߵ���__________ (�����ʽ)��ԭ����______________________________________________________________
(5)D��E���γɻ����ᄃ��ľ�����ͼ��ʾ ��
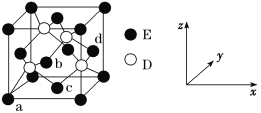
���ڸþ����У�E����λ��Ϊ________��
��ԭ����������ɱ�ʾ�����ڲ���ԭ�ӵ����λ�á���ͼ�����У�ԭ���������aΪ(0,0,0)��bΪ(1/2,0,1/2)��cΪ(1/2,1/2,0)����d���������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������С�մ�ˮ��Һ�ı�����ȷ���ǣ�������
A. ���ڵĵ���ƽ��ΪHCO3-��H2O![]() H2CO3��OH��
H2CO3��OH��
B. c��Na������c��H������c��HCO3-����c��CO32-����c��OH����
C. HCO3-�ĵ���̶ȴ���HCO3-��ˮ��̶�
D. c��Na������c��HCO3-����c��CO32-����c��H2CO3��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�ֺϳɇ��飨E����·�����£�
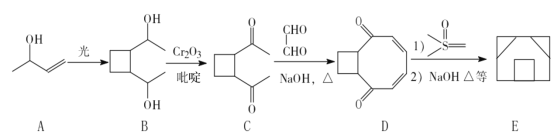
�� A�����������ŵ�������_______________��E�ķ���ʽΪ_________��
�� A��B��B��C�ķ�Ӧ���ͷֱ���___________��___________��
�� ��һ�������£�B����������ɷ���������Ӧ���仯ѧ����ʽΪ______________��
�� F��һ�ַ����廯�����ͬʱ��������������F��ͬ���칹����_____�֡�
�� 1��F���ӱ�1��C������������ԭ��
�� ��������3��ȡ����
�� 1molF����2molNaOH��Ӧ
д�����к˴Ź�������ͼ��5��壬�������Ϊ3��2��2��2��1��һ�����ʵĽṹ��ʽ��__________��
�� 1��2-��������( 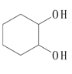 )��һ����Ҫ���л��ϳ�ԭ�ϣ���������еĺϳ�·�ߣ���
)��һ����Ҫ���л��ϳ�ԭ�ϣ���������еĺϳ�·�ߣ���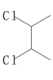 ��
��![]() Ϊ��Ҫԭ�ϣ���ƺϳ�1��2-���������ĺϳ�·�ߡ�______________
Ϊ��Ҫԭ�ϣ���ƺϳ�1��2-���������ĺϳ�·�ߡ�______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ���¶��£��������ݻ���Ϊ2.0 L�ĺ����ܱ������з�����Ӧ��2NO(g)��2CO(g)![]() N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ����ͼ��ʾ������˵����ȷ����
N2(g)��2CO2(g)������������ʼ���ʵ����뷴Ӧ�¶����±���ʾ����Ӧ�����мס���������CO2�����ʵ�����ʱ��仯��ϵ����ͼ��ʾ������˵����ȷ����
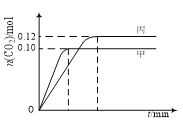
���� | �¶�/�� | ��ʼ���ʵ���/mol | |
NO (g) | CO (g) | ||
�� | T1 | 0.20 | 0.20 |
�� | T1 | 0.30 | 0.30 |
�� | T2 | 0.20 | 0.20 |
A. �÷�Ӧ������ӦΪ���ȷ�Ӧ
B. �ﵽƽ��ʱ������CO2����������ȼ��еĴ�
C. T1��ʱ������ʼʱ����г���0.40 mol NO��0.40mol CO��0.40mol N2��0.40mol CO2����Ӧ�ﵽ��ƽ��ǰv(��)��v(��)
D. T2��ʱ������ʼʱ����г���0.06mol N2��0.12 mol CO2�����ƽ��ʱN2��ת���ʴ���40%
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com