
ЁОЬтФПЁПВПЗжШѕЫсЕФЕчРыЦНКтГЃЪ§ШчБэЃК
ЃЈ1ЃЉЪвЮТЯТЂй0.1 molЁЄLЃ1 HCOONaЃЌЂк0.1 molЁЄLЃ1 NaClOЃЌЂл0.1 molЁЄLЃ1 Na2CO3ЃЌЂм0.1 molЁЄLЃ1
NaHCO3ШмвКЕФpHгЩДѓЕНаЁЕФЙиЯЕЮЊ_____________________________________ЁЃ
ЃЈ2ЃЉХЈЖШОљЮЊ0.1 molЁЄLЃ1ЕФNa2SO3КЭNa2CO3ЕФЛьКЯШмвКжаЃЌSO![]() ЁЂCO
ЁЂCO![]() ЁЂHSO
ЁЂHSO![]() ЁЂHCO
ЁЂHCO![]() ХЈЖШгЩДѓЕНаЁЕФЫГађЮЊ___________________________________ЁЃ
ХЈЖШгЩДѓЕНаЁЕФЫГађЮЊ___________________________________ЁЃ
ЃЈ3ЃЉЯТСаРызгЗНГЬЪНе§ШЗЕФЪЧ________(ЬюзжФИ)ЁЃ
a.2ClOЃЃЋH2OЃЋCO2===2HClOЃЋCO![]() b.2HCOOHЃЋCO
b.2HCOOHЃЋCO![]() ===2HCOOЃЃЋH2OЃЋCO2Ёќ
===2HCOOЃЃЋH2OЃЋCO2Ёќ
c.H2SO3ЃЋ2HCOOЃ===2HCOOHЃЋSO![]() d.Cl2ЃЋH2OЃЋ2CO
d.Cl2ЃЋH2OЃЋ2CO![]() ===2HCO
===2HCO![]() ЃЋClЃЃЋClOЃ
ЃЋClЃЃЋClOЃ
ЃЈ4ЃЉФГЮТЖШ(T Ёц)ЯТЕФШмвКжаЃЌc(HЃЋ)ЃН10ЃxmolЁЄLЃ1ЃЌc(OHЃ)ЃН10Ѓy molЁЄLЃ1ЃЌxгыyЕФЙиЯЕШчЭМЫљЪО.

Ђй ДЫЮТЖШЯТЃЌ0.01mol/LЕФNaOHШмвКжаЫЎЕчРыГіЕФOH-ХЈЖШЮЊ_____ЁЃ
ЂкдкДЫЮТЖШЯТЃЌ0.1 molЁЄLЃ1ЕФNaHSO4ШмвКгы0.1 molЁЄLЃ1ЕФBa(OH)2ШмвКАДЯТБэжаМзЁЂввЁЂБћЁЂЖЁВЛЭЌЗНЪНЛьКЯЃК
Мз | вв | Бћ | ЖЁ | |
0.1 molЁЄLЃ1Ba(OH)2ШмвКЬхЛ§/mL | 10 | 10 | 10 | 10 |
0.1 molЁЄLЃ1NaHSO4ШмвКЬхЛ§/mL | 5 | 10 | 15 | 20 |
АДЖЁЗНЪНЛьКЯКѓЃЌЫљЕУШмвКЯд________(ЬюЁАЫсЁБЁАМюЁБЛђЁАжаЁБ)адЃЎаДГіАДввЗНЪНЛьКЯКѓЃЌЗДгІЕФРызгЗНГЬЪНЃК__________________________ЁЃАДМзЗНЪНЛьКЯКѓЃЌЫљЕУШмвКЕФpHЮЊ________ЁЃ
ЁОД№АИЁП 3241 SO32->CO32-> HCO3- >HSO3- bd 10-10mol/L жаад жаад 11
ЁОНтЮіЁПЃЈ1ЃЉЫФжжбЮШмвКОљЮЊЧПМюШѕЫсбЮЃЌЫЎНтОљЯдМюадЃЌаЮГЩИУбЮЕФЫсдНШѕЃЌИУбЮЕФЫЎНтФмСІдНЧПЃЌМюадОЭдНЧПЃЛДгБэжаЕУГіЫсадДѓаЁЙиЯЕЮЊЃКHCOOH>H2CO3>HClO>HCO3-,ЫљвдбЮШмвКЕФpHгЩДѓЕНаЁЕФЙиЯЕЮЊЂл>Ђк>Ђм>ЂйЃЛе§ШЗД№АИЃКЂл>Ђк>Ђм>ЂйЁЃ
ЃЈ2ЃЉИљОнСНжжбЮШмвКОљЮЊЧПМюШѕЫсбЮЃЌЫЎНтОљЯдМюадЃЌаЮГЩИУбЮЕФЫсдНШѕЃЌИУбЮЕФЫЎНтФмСІдНЧПЃЌМюадОЭдНЧПЃЛДгБэжаЕУГіЫсадДѓаЁЙиЯЕЮЊЃКHSO3->HCO3-,ЫљвдNa2CO3ШмвКЫЎНтФмСІЧПЃЌЪЃгрЕФcЃЈCO![]() ЃЉХЈЖШаЁЃЌЩњГЩЕФc(HCO
ЃЉХЈЖШаЁЃЌЩњГЩЕФc(HCO![]() )ЖрЃЌвђДЫЫФжжРызгХЈЖШгЩДѓЕНаЁЕФЫГађЮЊЃКSO32->CO32-> HCO3- >HSO3- ЃЛе§ШЗД№АИЃКSO32->CO32-> HCO3- >HSO3-ЁЃ
)ЖрЃЌвђДЫЫФжжРызгХЈЖШгЩДѓЕНаЁЕФЫГађЮЊЃКSO32->CO32-> HCO3- >HSO3- ЃЛе§ШЗД№АИЃКSO32->CO32-> HCO3- >HSO3-ЁЃ
ЃЈ3ЃЉгЩгкЫсадH2CO3>HClO>HCO3-,ИљОнЧПЫсжЦБИШѕЫсЙцТЩЃЌClOЃЃЋH2OЃЋCO2===HClOЃЋHCO3-ЃЌaДэЮѓЃЛгЩгкЫсадHCOOH> H2CO3ЃЌИљОнЧПЫсжЦБИШѕЫсЙцТЩЃЌЗДгІПЩвдЗЂЩњЃЌbе§ШЗЃЛгЩгкЫсад.H2SO3> HCOOH> HSO3-ЃЌH2SO3ЃЋ HCOOЃ===HCOOHЃЋHSO3-ЃЌcДэЮѓЃЛгЩгкЫсадHCl>H2CO3>HClO>HCO3-,ИУЗДгІФмЙЛЗЂЩњЃЌdе§ШЗЃЛе§ШЗбЁЯюbdЁЃ
ЃЈ4ЃЉЂйИљОнЭМЯёПЩжЊЃКKW=10-12ЃЌИљОнЙЋЪНЃКЃЈc(OH-)(Мю)+ c(OH-)(ЫЎ)ЃЉЁСc(HЃЋ)ЃЈЫЎЃЉ= KW=10-12ЃЌЃЈ0.01+ c(OH-)(ЫЎ)ЃЉЁСc(HЃЋ)ЃЈЫЎЃЉ=10-12ЃЌгЩгкc(OH-)(ЫЎ)= c(HЃЋ)ЃЈЫЎЃЉЃЌЫљвдНќЫЦМЦЫуЕУЕНc(OH-)(ЫЎ)= c(HЃЋ)ЃЈЫЎЃЉ=10-10 molЁЄLЃ1ЃЛе§ШЗД№АИ10-10 molЁЄLЃ1ЁЃ
Ђк0.1 molЁЄLЃ1ЕФNaHSO4ШмвК20 mLКЭ0.1 molЁЄLЃ1ЕФBa(OH)2ШмвК10 mLЛьКЯКѓЃЌЧтРызгКЭЧтбѕИљРызгЧЁКУЭъШЋЗДгІЃЌЩњГЩСђЫсФЦЁЂСђЫсБЕКЭЫЎЃЌШмвКГЪжаадЃЛАДввЗНЪНЛьКЯКѓ
ЧтбѕИљРызггаЪЃгрЃЌШмвКЯдМюадЃЌРызгЗНГЬЪНЮЊBa2++SO42-+H++OH-=BaSO4Ё§+H2OЃЛМзЗНЪНЛьКЯКѓЃЌШмвКЯдМюадЃЌЪЃгрc(OH-)=ЃЈ0.1ЁС2ЁС10ЁС10-3-0.1ЁС5ЁС10-3ЃЉ/(10+5)ЁС10-3=0.1 molЁЄLЃ1,гЩгкKW=10-12ЃЌЫљвдc(HЃЋ)=10-11 molЁЄLЃ1, ЫљЕУШмвКЕФpHЮЊ11ЃЛе§ШЗД№АИЃКжаадЃЛ11ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗЧН№ЪєЕЅжЪ![]() ОШчЭМЫљЪОЕФЙ§ГЬзЊЛЏЮЊКЌбѕЫс
ОШчЭМЫљЪОЕФЙ§ГЬзЊЛЏЮЊКЌбѕЫс![]() ,вбжЊ
,вбжЊ![]() ЮЊЧПЫс,ЧыЛиД№ЯТСаЮЪЬт:
ЮЊЧПЫс,ЧыЛиД№ЯТСаЮЪЬт:
![]()
(1)Шє![]() дкГЃЮТЯТЮЊЙЬЬх,
дкГЃЮТЯТЮЊЙЬЬх, ![]() ЪЧФмЪЙЦЗКьШмвКЭЪЩЋЕФгаДЬМЄадЦјЮЖЕФЮоЩЋЦјЬхЁЃ
ЪЧФмЪЙЦЗКьШмвКЭЪЩЋЕФгаДЬМЄадЦјЮЖЕФЮоЩЋЦјЬхЁЃ
Ђй![]() ЕФЛЏбЇЪНЪЧ__________ЁЃ
ЕФЛЏбЇЪНЪЧ__________ЁЃ
ЂкдкЙЄвЕЩњВњжа, ![]() ЦјЬхЕФДѓСПХХЗХБЛгъЫЎЮќЪеКѓаЮГЩСЫ__________ЖјЮлШОСЫЛЗОГЁЃ
ЦјЬхЕФДѓСПХХЗХБЛгъЫЎЮќЪеКѓаЮГЩСЫ__________ЖјЮлШОСЫЛЗОГЁЃ
(2)Шє![]() дкГЃЮТЯТЮЊЦјЬх,
дкГЃЮТЯТЮЊЦјЬх, ![]() ЪЧКьзиЩЋЕФЦјЬхЁЃ
ЪЧКьзиЩЋЕФЦјЬхЁЃ
Ђй![]() ЕФЛЏбЇЪНЪЧ__________ЁЃ
ЕФЛЏбЇЪНЪЧ__________ЁЃ
Ђк![]() ЕФХЈШмвКдкГЃЮТЯТПЩгыЭЗДгІВЂЩњГЩ
ЕФХЈШмвКдкГЃЮТЯТПЩгыЭЗДгІВЂЩњГЩ![]() ЦјЬх,ЧыаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН________________ЁЃ
ЦјЬх,ЧыаДГіИУЗДгІЕФЛЏбЇЗНГЬЪН________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНЋ11.2gЬњЭЖШы200mLФГХЈЖШЕФбЮЫсжаЃЌЬњКЭбЮЫсЧЁКУЭъШЋЗДгІЁЃЧѓЃК
ЃЈ1ЃЉ11.2gЬњЕФЮяжЪЕФСП
ЃЈ2ЃЉЫљгУбЮЫсжаHClЕФЮяжЪЕФСПХЈЖШ
ЃЈ3ЃЉЗДгІжаЩњГЩЕФH2дкБъзМзДПіЯТЕФЬхЛ§
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМзЁЂввСНЗнЕШжЪСПЕФЬМЫсЧтФЦОЇЬхЃЌНЋМзгУлсліГфЗжМгШШКѓРфШДКѓЃЌНЋЙЬЬхЭъШЋзЊвЦЕНвЛЪдЙмжаЃЌ дйМгШызуСПЕФбЮЫсЃЛввВЛОМгШШжУгкСэвЛЪдЙмжаЃЌвВМгШызуСПЕФбЮЫсЁЃЗДгІЭъШЋКѓЃЌСНЪдЙмжаЪЕМЪВЮ МгЗДгІЕФHC1жЪСПжЎБШЮЊ( )
A. 2:3 B. 2:1 C. 1ЃК2 D. 1:1
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгаКЌгаЩйСПKC1ЁЂK2SO4ЁЂK2CO3дгжЪЕФKNO3ШмвКЃЌбЁдёЪЪЕБЕФЪдМСГ§ШЅдгжЪЃЌЕУЕНДПОЛЕФKNO3ЙЬЬхЃЌЪЕбщСїГЬШчЯТЭМЫљЪОЁЃ
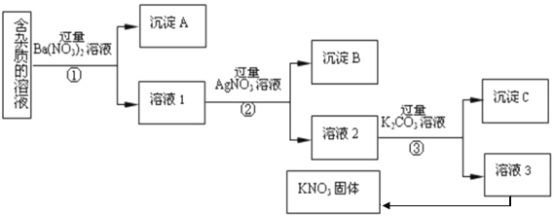
зЂЃКKNO3ЙЬЬхШнвзЪмШШЗжНт
(1)ГСЕэAЕФжївЊГЩЗжЪЧ___________ЁЂ___________(ЬюЛЏбЇЪН)ЃЛ
(2)ЮЊСЫГ§ШЅШмвК3жаЕФдгжЪЃЌПЩвдЯђЦфжаМгШыЪЪСПЕФ___________ЃЛГ§дгКѓДгШмвК3ЛёЕУKNO3ОЇЬхЕФВйзїЪЧ___________ЁЂ___________ЁЂЙ§ТЫЃЛ
(3)ВНжшЂлМгШыЙ§СПK2CO3ШмвКЕФФПЕФЪЧ___________ЃЛ
(4)ЪЕбщЪвгУЩЯЪіЪЕбщЛёЕУЕФKNO3ЬхХфжЦ450mL0.40 mol/L KNO3ШмвКЃЌашГЦШЁKNO3ЙЬЬхЕФжЪСПЪЧ_____gЃЛ
(5)ЯТСаВйзїЛсЕМжТЫљХфШмвКХЈЖШЦЋДѓЕФЪЧЃЈ___________ЃЉ
A.ЪЙгУСЫЩњатЕФэРТы
B.ЖЈШнЪБбіЪгПЬЖШЯп
C.ШнСПЦПЪЙгУЧАгУеєСѓЫЎЯДОЛЕЋУЛгаИЩдя
D.ЙЬЬхШмНтКѓЮДРфШДЕНЪвЮТОЭзЊвЦЕНШнСПЦПжа
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУЯТСаЪЕбщзАжУНјааЯргІЪЕбщЃЌЩшМЦе§ШЗЧвФмДяЕНЪЕбщФПЕФЕФЪЧ

A. МзгУгкЪЕбщЪвжЦШЁЩйСПCO2 B. ввгУгкХфжЦвЛЖЈЮяжЪЕФСПХЈЖШЕФСђЫс
C. БћгУгкФЃФтЩњЬњЕФЕчЛЏбЇИЏЪД D. ЖЁгУгкеєИЩA1Cl3ШмвКжЦБИЮоЫЎAlC13
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШЋЗАвКСїЕчГизАжУШчЭМЃЌЕчНтвКдкЕчНтжЪДЂЙоКЭЕчГиМфВЛЖЯбЛЗЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ
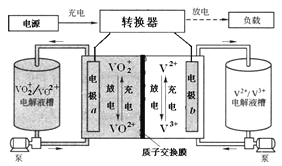
A. ГфЕчЪБЃЌЧтРызгЭЈЙ§НЛЛЛФЄвЦЯђгвВр
B. ГфЕчЪБЃЌЕчдДИКМЋСЌНгaЕчМЋ
C. ЗХЕчЪБзАжУЗЂЩњЕФзмЗДгІЮЊЃКVO2+ЃЋV2+ЃЋ2H+ЃНVO2+ЃЋV3+ЃЋH2O
D. жЪзгНЛЛЛФЄПЩзшжЙVO2+гыV2+жБНгЗЂЩњЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЭМгУЗжРрЗЈБэЪОСЫвЛаЉЮяжЪЛђИХФюжЎМфЕФДгЪєЛђАќКЌЙиЯЕЃЌВЛе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
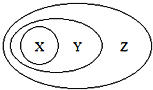
X | Y | Z | |
A | NaAlO2 | бЮ | ДПОЛЮя |
B | НКЬх | ЗжЩЂЯЕ | ЛьКЯЮя |
C | Al2O3 | СНадбѕЛЏЮя | бѕЛЏЮя |
D | ЕЅжЪВЮгыЗДгІ | жУЛЛЗДгІ | бѕЛЏЛЙдЗДгІ |
A. A B. B C. C D. D
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАБЦјМАКЌЕЊЛЏКЯЮядкЛЏЙЄЩњВњКЭЙњЗРЙЄвЕжаОпгаЙуЗКгІгУЁЃЧыЛиД№ЃК
ЃЈ1ЃЉвбжЊЃК(i)ЧтЦјЕФШМЩеШШЮЊ286.0 kJЁЄmol-1
(ii)4NH3(g)+3O2(g)![]() 2N2(g)+6H2O (l) ІЄH=- 1530.6 kJЁЄmol-1ЁЃ
2N2(g)+6H2O (l) ІЄH=- 1530.6 kJЁЄmol-1ЁЃ
КЯГЩАБЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ_____________________________ЁЃ
ЃЈ2ЃЉКуЮТКуШнЬѕМўЯТЃЌЦ№ЪМАДЮяжЪЕФСПжЎБШЮЊ1ЁУ1ЯђУмБеШнЦїжаГфШыN2(g)КЭH2(g)ЃЌЗЂЩњКЯГЩАБЕФЗДгІЁЃДяЦНКтКѓЃЌN2(g)ЕФЬхЛ§ЗжЪ§ЮЊ_______________ЃЛШЛКѓжЛНЕЕЭЮТЖШЃЌN2(g)ЕФЬхЛ§ЗжЪ§Лс_________ЃЈЬюбЁЯюзжФИЃЉЁЃ
A.діДѓ B.МѕаЁ C.ВЛБф D.ВЛФмХаЖЯ
ЃЈ3ЃЉTЁцЪБЃЌCO2(g)КЭNH3(g)КЯГЩФђЫиЕФдРэЮЊ2NH3(g)+CO2(g)![]() CO(NH2)2(s)+H2O(1)ЁЃдк2 LКуШнУмБеШнЦїжаЃЌЭЈШы1.2 mol NH3(g)КЭ0.6 mol CO2(g)ЃЌ2 minЪБЗДгІЧЁКУДяЕНЦНКтЃЌВтЕУc(NH3)=0.2 molЁЄL-1ЁЃ
CO(NH2)2(s)+H2O(1)ЁЃдк2 LКуШнУмБеШнЦїжаЃЌЭЈШы1.2 mol NH3(g)КЭ0.6 mol CO2(g)ЃЌ2 minЪБЗДгІЧЁКУДяЕНЦНКтЃЌВтЕУc(NH3)=0.2 molЁЄL-1ЁЃ
Ђй0~2minФкЃЌгУNH3БэЪОЕФЗДгІЫйТЪІд(NH3)=___________ЃЛЗДгІЕФЦНКтГЃЪ§K=____________ЁЃ
ЂкШєЦфЫћЬѕМўВЛБфЃЌ2 minЪБНЋШнЦїЬхЛ§бИЫйбЙЫѕЕН1LЃЌдк3 minЪБжиаТДяЕНЦНКтЃЌЧыдкЭМ1жаЛГі2~3 minФкc(NH3)ЫцЪБМф(t)БфЛЏЕФЧњЯпЙиЯЕЭМЁЃ

ЃЈ4ЃЉМюадАБЦјШМСЯЕчГиЕФзАжУШчЭМ2 ЫљЪОЃЌаДГіИКМЋЕФЕчМЋЗДгІЪН____________________ЁЃЕБЕчТЗжаУПЭЈЙ§3.6 mol e-ЃЌдђашвЊБъПіЯТПеЦјЕФЬхЛ§________________LЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com