
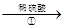 ����Һ
����Һ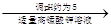
 ��Һ
��Һ
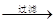 ��Һ
��Һ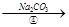
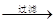 �˱�
�˱�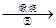 ZnO
ZnO


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
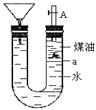
| A���˼���������Ǽػ��� |
| B����Ӧһ��ʱ��ú�ͻ�ȼ�� |
| C������������˶���������ˮ��Ӧ���������� |
| D�����ǽ�������ܹ۲쵽�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ��Ŀ�� | ʵ�鷽�� | ���ͻ���� |
| A | ����CH2=CH-CHO�к�̼̼˫�� | ����ϩȩ��Һ������ˮ�У���ˮ��ɫ | ��ϩȩ��̼̼˫�����嵥�ʷ����˼ӳɷ�Ӧ |
| B | ȷ��ij����Ũ��Һ������ | ��պ��Ũ��ˮ�IJ����������Լ�ƿ�ڣ��д������� | ������һ��Ϊ���� |
| C | ����һ�ݺ���ɫ����ɷ� | ʪ��ĵ��۵⻯����ֽ���������У���ֽ���� | ������һ��ΪBr2 |
| D | ̽����֬���������ˮ������� | ��֬����������м���NaOH��Һ���Ⱥ��ٷֲ� | ��֬�������������������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A���ζ��յ����ʱ���Ӷ��� | B����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ |
| C����ƿˮϴ��δ���� | D������NaOH�����л���Na2CO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢ڢܢݢۢ� | B���٢ܢڢۢݢ� |
| C���٢ܢڢޢۢ� | D���٢ݢۢܢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
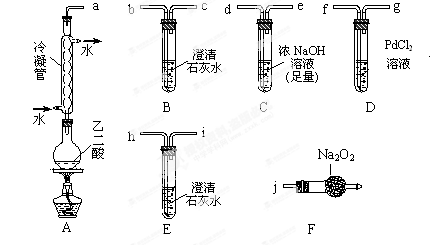
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A������ | B���ؽᾧ | C������ | D����ȡ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
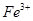 ����ܺ���
����ܺ���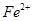 ����Ҫȷ�����е�
����Ҫȷ�����е�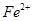 ��Ӧѡ�� ѡ����ţ���
��Ӧѡ�� ѡ����ţ���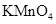 ��Һ
��Һ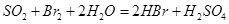 ��Ȼ���������
��Ȼ���������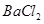 ��Һ�����ʵ�������ø������2��33g�����ڴ���֪����Y��
��Һ�����ʵ�������ø������2��33g�����ڴ���֪����Y��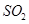 ���������Ϊ ��
���������Ϊ ��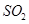 ��������Ľ������ͬѧ��Ϊ����Y�л����ܺ�����
��������Ľ������ͬѧ��Ϊ����Y�л����ܺ�����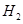 ��Q���塣Ϊ�����������̽��ʵ��״�ã�ͼ�мг�����ʡ�ԣ���
��Q���塣Ϊ�����������̽��ʵ��״�ã�ͼ�мг�����ʡ�ԣ���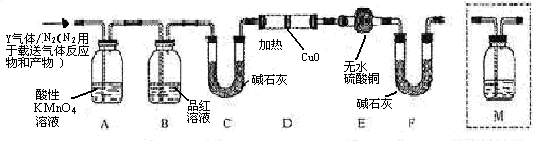
 ��Ԥ��ʵ������Ӧ�� ��
��Ԥ��ʵ������Ӧ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 O2(g) �� H2O(g) ��H����241.8 kJ��mol��1
O2(g) �� H2O(g) ��H����241.8 kJ��mol��1�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com