
����Ŀ��������һ�����͵���ɫ��Դ������һ����Ҫ�Ļ���ԭ�ϡ�
��1������ȼ����ֵ�ߡ�ʵ����,�ڳ��³�ѹ��1gH2��ȫȼ������Һ̬ˮ,�ų�142.9kJ���������ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ_____________________������֪��![]() ,�����ڿ�����ȼ������Һ̬ˮ�͵���ʱ���Ȼ�ѧ����ʽΪ______________________��
,�����ڿ�����ȼ������Һ̬ˮ�͵���ʱ���Ȼ�ѧ����ʽΪ______________________��
��2�������Ǻϳɰ�����Ҫԭ�ϡ�
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ�������(���ı�![]() ��
��![]() ����)����Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
����)����Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
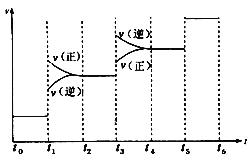
ͼ��t1ʱ����ƽ���ƶ�������������_______________,���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����______________��
�ڰ����������������ᣬ�˹������漰���������![]() �ȡ����ڷ�Ӧ��
�ȡ����ڷ�Ӧ��![]() ,���¶�Ϊ
,���¶�Ϊ![]() ʱ,ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
ʱ,ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ��
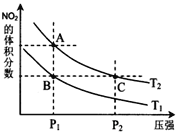
����˵����ȷ����______��
a��![]() ����Ļ�ѧƽ�ⳣ����
����Ļ�ѧƽ�ⳣ����![]()
b��![]() �����������ɫ��
�����������ɫ��![]() dz��
dz��![]() ��
��
c��![]() ���������ƽ����Է���������
���������ƽ����Է���������![]()
d��![]() ����ķ�Ӧ���ʣ�
����ķ�Ӧ���ʣ�![]()
e����״̬B��״̬A�������ü��ȵķ���
���𰸡�H2(g)+1/2O2(g)=H2O(l) H����285.8kJ/mol 4NH3(g)+3O2(g)=2N2(g)+6H2O(l) H����1530kJ/mol ��ѹ t2��t3 bde
��������
(1)1gH2��ȫȼ������Һ̬ˮ,�ų�142.9kJ��������1mol������2g������ȫȼ������Һ̬ˮ,�ų�142.9kJ��2��������˱�ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪH2(g)+1/2O2(g)=H2O(l) H����285.8kJ/mol �١�����Ϊ![]() �ڣ���˸��ݸ�˹���ɿ�֪�١�6���ڡ�2���õ�4NH3(g)+3O2(g)=2N2(g)+6H2O(l) H����1530kJ/mol��
�ڣ���˸��ݸ�˹���ɿ�֪�١�6���ڡ�2���õ�4NH3(g)+3O2(g)=2N2(g)+6H2O(l) H����1530kJ/mol��
(2)������Ӧ�������С�ķ��ȷ�Ӧ����ͼ�������t1ʱ���淴Ӧ���ʾ���������Ӧ���ʴ����淴Ӧ���ʣ�������Ӧ������У����ƽ���ƶ��������Ǽ�ѹ��t3ʱ���淴Ӧ���ʾ������淴Ӧ���ʴ�������Ӧ���ʣ����淴Ӧ������У����ƽ���ƶ������������¡���ѹƽ�������ƶ���NH3�ĺ�����������ʱƽ�������ƶ���NH3�ļ�С�����Ա�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����t2��t3��
��a��![]() �����Ӧ���¶���ͬ����ѧƽ�ⳣ����ȣ�a����
�����Ӧ���¶���ͬ����ѧƽ�ⳣ����ȣ�a����
b��A�������ѹǿС��C�������ѹǿ��ѹǿ�������Ũ�ȴ���ɫ����������������ɫ��![]() dz��
dz��![]() ���ȷ��
���ȷ��
c����ͼ�п��Կ�����![]() ����NO2�����������ͬ������ƽ����Է���������ȣ�����
����NO2�����������ͬ������ƽ����Է���������ȣ�����
d��ѹǿA��С��C�㣬��������ķ�Ӧ���ʣ�![]() ����ȷ��
����ȷ��
e����״̬B��״̬A��NO2������������ӣ�ƽ��������Ӧ������У���˿����ü��ȵķ�������ȷ��
��Ϊbde��


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С�齫������SO2���建����ͨ�뵽ʢ��25mL0.1mol�� L��1��Ba(NO3)2��Һ�У��õ�BaSO4������Ϊ̽���÷�Ӧ�е�����������С����������¼��裺
����������Һ�е�NO3����
��������________________��
(1)��С�����������ʵ����֤�˼���������(Ϊ�ų��������Լ������ĸ��ţ�����������ʵ��������Һʱ��Ӧ___________________)������д�±���
ʵ�鲽�� | ʵ������ | ���� | |
ʵ���� | ��ʢ��25mL0.1mol��L��1BaCl2��Һ���ձ��л���ͨ�봿����SO2���� | ______ | ���������� |
ʵ���� | ��ʢ��25mL0.1mol�� L��1Ba(NO3)2��Һ���ձ��л���ͨ�봿����SO2���� | ______ | |
(2)Ϊ�����о��÷�Ӧ����С�黹�����������ʵ������Һ��pH��ͨ��SO2����ı仯������ͼ��V1ʱ��ʵ��������ҺpHС��ʵ������ԭ����(�����ӷ���ʽ��ʾ)��________��
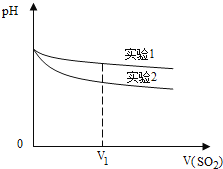
(3)��֤��������ijͬѧ��������·�������������б���(���Բ�����)��
ʵ�鲽�� | ʵ������ | ʵ��Ŀ�� | ||
ʵ���� | ͬʵ�������� | ͬʵ������������� | ______ | |
ʵ���� | ______ | ______ | ______ | |
(4)������֪��H2SO3�Ƕ�Ԫ��(Kl=1.54��10��2��K2=1.02��10��7)�������ʵ�鷽����֤H2SO3�Ƕ�Ԫ��______(�Լ���������ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Դ�����ö�����̼�����ɼ�������������ŷţ��������»��ȼ�ϻ���Ҫ��ҵ��Ʒ��
(1)��CO2��NH3Ϊԭ�Ͽɺϳɻ�������[CO(NH2)2]��
��֪����2NH3(g)��CO2(g)��NH2CO2NH4(s)��H ����159.47 kJ��mol-1
��NH2CO2NH4(s)��CO(NH2)2(s)��H2O(g)��H ��+116.49 kJ��mol-1
��H2O(l)��H2O(g)��H ��+88.0 kJ��mol-1
��д��NH3��CO2�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ______________��
(2)��һ�������£�������̼ת��Ϊ����ķ�Ӧ���£�CO2(g)+4H2(g)![]() CH4(g)+2H2O(g) ��H��0
CH4(g)+2H2O(g) ��H��0
����һ�ݻ�Ϊ2L�ĺ����ܱ������г���һ������CO2��H2����300��ʱ����������Ӧ���ﵽƽ��ʱ�����ʵ�Ũ�ȷֱ�ΪCO2��0.2mol��L-1��H2��0.8mol��L-1��CH4��0.8mol��L-1��H2O��1.6mol��L-1����ʼ����CO2��H2�����ʵ����ֱ�Ϊ_____��_____��CO2��ƽ��ת����Ϊ______��
������������ͬ�ĺ��ݾ���(�����û����������)�ܱ�����I��II����I�г���1 molCO2,��4 molH2����II�г���1 mol CH4��2 mol H2 O(g)��300���¿�ʼ��Ӧ���ﵽƽ��ʱ������˵����ȷ����_________(����ĸ)��
A������I��II������Ӧ������ͬ B������I��II��CH4�����ʵ���������ͬ C������I��CO2�����ʵ���������II�еĶ� D������I��CO2��ת����������II��CH4��ת����֮��С��1
(3)��ʢ�ٴ�ѧ���о���Ա�о���һ�ַ�������ʵ��ˮ������ʱCO2���ŷţ������ԭ����ͼ��ʾ��
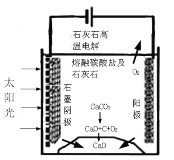
�������������̵�����ת����ʽ��_____��
��������ⷴӦ���¶�С��900��ʱ����̼����ȷֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ___�������ĵ缫��ӦʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���о�![]() �ȴ�����Ⱦ����Ĵ���������Ҫ���塣
�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1����֪��![]() ��
��![]() ��
��
��Ӧ![]() ��
��![]() ��________��
��________��
��2��һ�������£���![]() ��
��![]() �������
�������![]() �����ܱ������з���������Ӧ�����������Ӧƽ��ʱ
�����ܱ������з���������Ӧ�����������Ӧƽ��ʱ![]() ��
��![]() �����Ϊ
�����Ϊ![]() ����ƽ�ⳣ��
����ƽ�ⳣ��![]() ��________��������λС������
��________��������λС������
��3��![]() �����ںϳɼ״�����Ӧ����ʽΪ
�����ںϳɼ״�����Ӧ����ʽΪ![]() ��
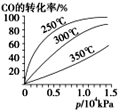
��![]() �ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ���÷�Ӧ
�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ���÷�Ӧ![]() ________
________![]() (���������)��ʵ����������������
(���������)��ʵ����������������![]() ��
��![]() ���ң�ѡ���ѹǿ��������__________________��
���ң�ѡ���ѹǿ��������__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ԭ������Ϊ1��18��Ԫ����(�û�ѧʽ��д)��
(1)��ˮ��Ӧ����ҵĽ���������________��
(2)��ˮ��Ӧ����ҵķǽ���������________��
(3)������������ɫ�����嵥����______��__________��
(4)�ڿ�����������ȼ�ĵ�����________��
(5)��ϡ������Ԫ���⣬ԭ�Ӱ뾶����Ԫ����_________������ԭ�ӽṹʾ��ͼ��_______��
(6)ԭ�Ӱ뾶��С��Ԫ����______�������_____________________��
(7)��̬�⻯���ˮ��Һ�ʼ��Ե�Ԫ����________��
(8)���ȶ�����̬�⻯��Ļ�ѧʽ��________��
(9)����������Ӧˮ�����������ǿ��Ԫ����_________��
(10)�ǽ���Ԫ�ص���̬�⻯���к�������������ߵ�Ԫ����____����������������С����̬�⻯��Ļ�ѧʽ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I������(H2C2O4)���������������������ܹ�������Ӧ��
��ʵ��1����ͬѧ��8.00 mL 0.001 mol/L KMnO4��Һ��5.00 mL 0.01 mol/L H2C2O4��Һ��Ӧ���о���ͬ�����Ի�ѧ��Ӧ���ʵ�Ӱ�졣�ı���������£�
��� | KMnO4��Һ /mL | H2C2O4��Һ /mL | 10%�������/mL | �¶�/�� | �������� |
�� | 8.00 | 5.00 | 3.00 | 20 | |
�� | 8.00 | 5.00 | 3.00 | 30 | |
�� | 8.00 | 5.00 | 1.00 | 20 | 2.00 mL����ˮ |
��1��д������(H2C2O4)����������Һ�����������·�Ӧ�����ӷ���ʽ________��
��2����������ʵ����Ŀ����̽��__________�Ի�ѧ��Ӧ���ʵ�Ӱ�졣
��ʵ��2����ͬѧ���о������������������������·�Ӧ��Ӱ������ʱ����,���������Ը��������Һ��ʼһ��ʱ�䷴Ӧ���ʽ���,��Һ��ɫ������,�����ú�ͻȻ��ɫ,��Ӧ�������Լӿ졣
��3�������������,��ͬѧ��Ϊ�����������ط�Ӧ����,������Һ�¶�����,��Ӧ���ʼӿ졣��Ӱ�컯ѧ��Ӧ���ʵ����ؿ�,����뻹������_______��Ӱ�졣
��4������ʵ��֤����IJ���,�������Ը��������Һ�Ͳ�����Һ��,����Ҫѡ����Լ����������____������ĸ����
a������� b��ˮ c���������� d��������
������ͼ��ʾ��װ�ý����к��ȵIJⶨʵ�飬�ֱ�ȡ![]() ��
��![]() ��Һ��
��Һ��![]() ���������ʵ�飬�ش��������⣺
���������ʵ�飬�ش��������⣺
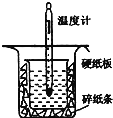
��1������ͼʵ��װ�ÿ���������ȱ�ٵ�һ�ֲ�����Ʒ��__________������֮�⣬װ���е�һ�����Դ�����__________��
��2��������Ϊ![]() ��NaOH��Һ��
��NaOH��Һ��![]() ��������Һ���ܶȶ���
��������Һ���ܶȶ���![]() ���кͺ�������Һ�ı�����
���кͺ�������Һ�ı�����![]() ��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
��ͨ���������ݼ����к��ȡ�H=__________���������С�����һλ����
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | ||
H2SO4 | NaOH | ƽ��ֵ | ||
1 | 26.2 | 26.0 | 26.1 | 29.5 |
2 | 27.0 | 27.4 | 27.2 | 32.3 |
3 | 25.9 | 25.9 | 25.9 | 29.2 |
4 | 26.4 | 26.2 | 26.3 | 29.8 |
��3������ʵ����ֵ�����![]() ��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
��ƫ�����ƫ���ԭ������ǣ�����ĸ��_____��
a��ʵ��װ�ñ��¡�����Ч����
b�����¶ȼƲⶨ![]() ��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
c���ֶ�ΰ�![]() ��Һ����ʢ�������С�ձ���
��Һ����ʢ�������С�ձ���
d����������ʵ������¶Ⱦ��������ƽ��ֵ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£����и���������ָ����Һ���ܴ����������(����)
A.pH��2����Һ��Na����Fe2����I����NO3-
B.c(AlO2-)��0.1 mol��L��1����Һ��K����Na����OH����SO42-
C.![]() ��0.1 mol��L��1����Һ��Na����NH4+��SiO32-��ClO��
��0.1 mol��L��1����Һ��Na����NH4+��SiO32-��ClO��
D.c(Fe3��)��0.1 mol��L��1����Һ��Mg2����NH4+��Cl����SCN��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ԫ��֮һ���ҹ���ǰ��ʳ���м�KI�ӹ����Ρ�
(1) Ŀǰ�ӵ�ʳ���У�����KI����Ҫԭ����__________________________��
(2) ��Fe3I8���뵽K2CO3��Һ�У�����Fe3O4��KI��һ�����壬�÷�Ӧ�Ļ�ѧ����ʽΪ__________��
(3) ȷ��ȡijKI��Ʒ3.500 0 g���Ƴ�100.00 mL��Һ��ȡ25.00 mL������Һ������ƿ�У�����15.00 mL 0.100 0 mol��L��1 K2Cr2O7������Һ(Cr2O72-ת��ΪCr3��)����ַ�Ӧ����г�ȥ���ɵ�I2����ȴ��������KI����0.200 0 mol��L��1 Na2S2O3����Һ�ζ����յ�(I2��S2O32-��Ӧ����I����S4O62-)������Na2S2O3����Һ24.00 mL���������Ʒ��KI����������____________ (д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С��Ӧ����ͼ��ʾ�ķ����о����ʵ����ʣ���������E����Ҫ�ɷ��������������ǿ�����ˮ�������ش��������⣺

��1�������о���ʵ�飩����ҪĿ����________��
��2��ŨH2SO4��������_________��
��3�����������ʵķ���������������ʵ����ƴ����¹��������¹�������________������ͼ�е�D����ͼ�����ʽ���������¹������Ĵ�ʩ_______�����з�����Ӧ�Ļ�ѧ����ʽΪ__________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com