
����Ŀ����ú��ʯ���п�������������ԭ��A��B��A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ��B��һ�ֱ�ˮ�����״Һ̬����0.1 mol��������������������ȫȼ�գ�����0.6 mol CO2��0.3 molH2O���ش��������⣺
(1)A�ĵ���ʽ________��B�Ľṹ��ʽ________��
(2)��������A��B��ȫȼ��ʱ����O2�����ʵ���A_______B(������������������������)
(3)��A���ڵ�ͬϵ��Cʹ������Ȼ�̼��Һ��ɫ�Ļ�ѧ��Ӧ����ʽ��_________________����Ӧ���ͣ�______________��
���𰸡�![]()
![]() �� CH2=CHCH3��Br2��CH2BrCHBrCH3 �ӳɷ�Ӧ
�� CH2=CHCH3��Br2��CH2BrCHBrCH3 �ӳɷ�Ӧ
��������
A��һ�ֹ�ʵ����������IJ�����������һ�����ҵ�ʯ�ͻ�����չˮƽ����AΪCH2=CH2��B��һ�ֱ�ˮ�����״Һ̬����0.1mol��������������������ȫȼ�գ�����0.6mol CO2��0.3molˮ��������N��C��= 0.6mol/0.1mol=6��N��H��= 0.3mol��2/0.1mol=6����B�ķ���ʽΪC6H6��B����Է�������Ϊ78����12n+2n-6=78�����n=6������BΪ����
��1��AΪ��ϩ���ṹ��ʽΪCH2=CH2������ʽΪ��![]() ��BΪ�����ṹ��ʽΪ
��BΪ�����ṹ��ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]() ��
��
��2����ϩ��HԪ�����������ȱ���HԪ��������������ͬ��������ϩ����ȼ�գ���ϩ���ĵ��������࣬����������A��B��ȫȼ��ʱ����O2�����ʵ���A��B���ʴ�Ϊ������
��3����A���ڵ�ͬϵ��CΪCH2=CHCH3��CH2=CHCH3����ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��CH2=CHCH3+Br2��CH2BrCHBrCH3���ʴ�Ϊ��CH2=CHCH3+Br2��CH2BrCHBrCH3���ӳɷ�Ӧ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�����������˵����ȷ����(����)
�ٱ�״���£�11.2 L�����������ϵĵ���������������ԭ����ΪNA
��ͬ��ͬѹ�£������ͬ����������������ķ��������
��1 L 2 mol��L��1��AlCl3��Һ�к�������Ϊ6NA
�ܱ�״���£�22.4 L H2O�з�����ΪNA
��32 g O2��O3��������к���ԭ����Ϊ2NA
A. �٢ڢۢ� B. �٢ڢۢ�
C. �٢ۢ� D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ����
A. ϡ������Ƚ��ȶ������������������ԭ������㶼����8�����ȶ��ṹ
B. Ԫ�����ڱ������塢���塢����ȹ�16����
C. �������ڵ�Fe��Co��Ni��λ�ڵ�VIIIB�壬���Ի�ѧ��������
D. P3-��ӦԪ��λ�ڵ������ڵ�VA��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E��F�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������A��̬ԭ���У�����ռ�ݵ�����ܲ����ΪL������������ԳɶԵ��ӣ�����̬�����4���ɵ����ӣ�C�Ļ�̬ԭ���е���ռ�ݵ�����ܲ����ҲΪL��p�ܼ�����4�����ӣ�Dԭ�ӵ���������������Ӳ���֮������A��B��C����Ԫ�ص�ԭ������֮�ͣ�Eԭ�Ӻ��������������ԭ�Ӷ�������F��̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ���ش��������⣺
(1)��̬F��������Ų�ʽΪ______________________��
(2)��̬Eԭ�ӵļ۵����Ų�ʽΪ______________________��
(3)DC4���Ŀռ乹����___________����DC4����Ϊ�ȵ������һ�ַ���Ϊ___________(�ѧʽ)��HBC3���Ա�HBC2ǿ����ԭ����______________________��
(4)A��B��C����Ԫ�ص�һ������������___________(��Ԫ�ط��ű�ʾ)����ԭ����_________________________________��
(5)��E(NO3)2��Һ����μ��백ˮ���տ�ʼʱ������ɫ��E(OH)3����������ˮ����ʱ���������ܽ⣬����[E(NH3)6]2+����ɫ��Һ����1mol[E(NH3)6]2+���е�������ĿΪ___________��
(6)D��Na�γɵ�һ�����ӻ��������ͼ��ʾ�����ṹͼ�к����ʾNa��λ�ã������ʾD��λ�ã���֪�þ����ı߳�Ϊncm�������ӵ�����ΪNA���������ܶ���=___________g/cm3(�ú�n��NA�ļ���ʽ��ʾ)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���W������������߷��Ӳ��Ϻϳɵ��м���ȣ��Ʊ�W��һ�ֺϳ�·�����¡�

��ش��������⣺
��1��F�Ļ�ѧ������_______________���ٵķ�Ӧ������_______________��
��2��B�к��еĹ�������_______________��д���ƣ���
��3��D�ۺ����ɸ߷��ӻ�����Ļ�ѧ����ʽΪ________________��
��4����Ӧ�۵Ļ�ѧ����ʽ��___________________��
��5�����㻯����N��A��ͬ���칹�壬���к˴Ź�������Ϊ�����Ľṹ��ʽΪ_________________��
��6������ʽC9H10O2���л����ṹ�к��б����ҿ����뱥��NaHCO3��Һ��Ӧ�ų������ͬ���칹����__________________��(�����������칹)��
��7�������л���W�������ϳ�·�ߣ������MΪ��ʼԭ���Ʊ�F�ĺϳ�·��(���Լ���ѡ)��____________
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʹ������к͵ζ����ⶨ���۰״�(��Ҫ�ɷ���CH3COOH)��������(g��100 mL��1)����֪CH3COOH + NaOH===CH3COONa + H2O �յ�ʱ������Һ�ʼ��ԡ�
��.ʵ�鲽�裺
(1)����Һ����ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL__________(����������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
(2)��_____ȡ����״���Һ20.00 mL����ƿ�С�
(3)�μ�2��_____________��ָʾ����
(4)��ȡʢװ0.100 0 mol��L��1NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ������ ���Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
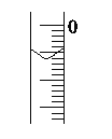
(5)�ζ�����__________________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼
ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
V(��Ʒ) | 20.00 | 20.00 | 20.00 | 20.00 |
V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.���ݴ��������ۣ�
(1)�����������ݷ�����c(���۰״�)��________mol��L��1��
(2)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫС����_______(��д���)��
A����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
B����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
C����ƿ�м������״���Һ���ټ�����ˮ
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
E. �ζ��յ����ʱ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д�����и�����������ƣ���д���б�ŵ��������ơ�

A����������______________����������______________
B����������______________����������______________
C����������______________����������___________��______________��____________
D����������_____________����������________________
��2������100ml��3.00mol/L NaCl��Һ��
�ټ�����ҪNaCl���������__________g��
�ڸ��ݼ���������������ƽ�Ƴ���NaCl����__________g��
�۽��ƺõ�NaCl��������ձ��У�����������ˮ�ܽ⡣
�ܽ��ձ��е���Һע������ƿ��������������ˮ_________________2~3�Σ�__________Ҳ��ע������ƿ������ҡ������ƿ��ʹ��Һ�����
�ݽ�����ˮע������ƿ��Һ��������ƿ���̶�����________ʱ������______________�μ�����ˮʹҺ����̶������У��Ǻ�ƿ�������µߵ���ҡ�ȡ�
��������Һ��
��3��ʵ�������ò�����������Ͳ���________________________________________��
��4��ΪʲôҪ������ˮϴ���ձ�������ϴ��ҺҲע������ƿ����____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�ij��Ӧ��ƽ�⣬ƽ�ⳣ��K=![]() ������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ����
������ʱ���¶����ߣ�H2Ũ�ȼ�С������˵����ȷ����
A���÷�Ӧ���ʱ�Ϊ��ֵ
B�����º����£�����ѹǿ��H2Ũ��һ����С
C�������¶ȣ��淴Ӧ���ʼ�С
D���÷�Ӧ��ѧ����ʽΪCO+H2O=CO2+H2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺

��1�����Ȼ�����Һ�еõ��Ȼ��ع��壬ѡ��װ��___�������װ��ͼ����ĸ����ͬ������ȥ����ˮ�е�Cl-�����ʣ�ѡ��װ��___����������ˮ��Cl-�Ƿ�����ķ�����____��
��2���ӵ�ˮ�з����I2��ѡ��װ��___���÷��뷽��������Ϊ____��
��3��װ��A�Тٵ�������____����ˮ�ķ����Ǵ�___�ڽ�ˮ��װ��B�ڷ�ҺʱΪʹҺ��˳���µΣ�Ӧ���еľ��������____��
��4����ˮ���̲��ŷḻ����Դ����ʵ������ȡ������ˮ��������ͼ���̵�ʵ�飺
![]()
�����к�Ca2+��Mg2+��Fe3+��SO42-�����ʣ���Ҫ�ᴿ������ۺ����á������ᴿ�IJ����У�
�ټ��������Na2CO3��Һ�� �ڼ��������BaCl2��Һ���ۼ��������NaOH��Һ�� �ܵ�����Һ��pH����7�� ���ܽ⣻ ���ˣ� ��������
��ȷ�IJ���˳����____����д��ţ���
a���ݢڢۢ٢ޢܢ� b���ݢ٢ڢۢޢܢ�
c���ݢڢ٢ۢܢޢ� d���ݢۢڢ٢ޢܢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com