
����Ŀ����20����60����Ժ����Ƿ�����120���ֺ������![]() ��
��![]() ��
��![]() ��
��![]() ��
��![]() ��ø�͵����ʡ����Ǵ����������������ϵ���������֮һ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ�����������ʵ�飺
��ø�͵����ʡ����Ǵ����������������ϵ���������֮һ��ij��ѧ��ȤС�����о�ij����ؽṹ�����ʱ�����������ʵ�飺
ʵ��һ���ⶨ���������
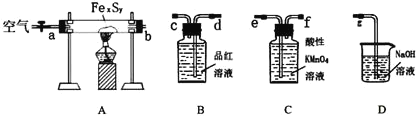
(1)����װ�ã�����д�ӿ�˳��b��____________________
(2)���װ�õ������ԣ���A�з���0.4g����ص���Ʒ![]() ���в�����ˮ�����������
���в�����ˮ�����������![]() ,��B�м���Ʒ����Һ����C�м���30mL 0.1mol/L������
,��B�м���Ʒ����Һ����C�м���30mL 0.1mol/L������![]() ��Һ��
��Һ��
(3)ͨ����������ȣ����ֹ�����ת��Ϊ����ɫ��
(4)��������ȫת����ȡC�е�![]() ��Һ
��Һ![]() ����0.1mol/L�ĵ⻯��
����0.1mol/L�ĵ⻯��![]() ��Һ���еζ�����¼�������£�
��Һ���еζ�����¼�������£�
�ζ����� | ������Һ��� | ���ĵ⻯����Һ��� | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 |
|
|
|
2 |
|
|
|
3 |
|
|
|
ʵ������ⶨ����������
ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�0.32g���壮�Իش��������⣺
(1)�����ʵ��һ����װ��A�������Եķ�����_________
(2)�ζ��յ���жϷ�����_________
(3)װ��B��Ʒ����Һ��������_______![]() ��ͬѧ�������ȥBװ�ã���ʵ��û��Ӱ�죬��Ŀ�����______
��ͬѧ�������ȥBװ�ã���ʵ��û��Ӱ�죬��Ŀ�����______![]() ѡ����������������������
ѡ����������������������![]() ��������_________
��������_________
(4)��KI��Һ�ζ�![]() ��Һʱ������Ӧ�����ӷ���ʽΪ_________
��Һʱ������Ӧ�����ӷ���ʽΪ_________
(5)�������������ؽṹ�Ļ�ѧʽ_________
(6)���в�������������![]() ƫ�����_________
ƫ�����_________
![]() �ζ�ʣ��
�ζ�ʣ��![]() ��Һʱ��KI��Һ�ε���ƿ���һ��
��Һʱ��KI��Һ�ε���ƿ���һ��
![]() ����KI��Һʱ������ʱ���ӿ̶���
����KI��Һʱ������ʱ���ӿ̶���
![]() �õ⻯����Һ�ζ�ʣ��
�õ⻯����Һ�ζ�ʣ��![]() ��Һʱ���ζ�ǰ�����ݣ��ζ���������
��Һʱ���ζ�ǰ�����ݣ��ζ���������
![]() ʵ����У����������ղ����
ʵ����У����������ղ����
���𰸡�b��![]() д��д����
д��д����![]() �ڵ���b���ϳ����ܣ���ĩ�˲���ˮ���У��رջ������þƾ�����Ӳ���Թ�A�����ܳ����ܿ������ݲ�������ȥ�ƾ��ƣ������γ�һ��ˮ����˵��װ������������ �������һ��KI��Һ����Һ��ɫ��ȥ���Ұ���Ӳ��ָ�Ϊ��ɫ ������������Ƿ����Ը��������ȫ���� ���� ��B�и��������Һ����ɫ����ȥ��˵����������������ȫ
�ڵ���b���ϳ����ܣ���ĩ�˲���ˮ���У��رջ������þƾ�����Ӳ���Թ�A�����ܳ����ܿ������ݲ�������ȥ�ƾ��ƣ������γ�һ��ˮ����˵��װ������������ �������һ��KI��Һ����Һ��ɫ��ȥ���Ұ���Ӳ��ָ�Ϊ��ɫ ������������Ƿ����Ը��������ȫ���� ���� ��B�и��������Һ����ɫ����ȥ��˵����������������ȫ ![]()
![]()
![]()
��������
����ص���Ʒ��װ������O2��Ӧ���õ�SO2�����SO2�ĺ����������Ը���������գ�����Ʒ����Һ���SO2�Ƿ�������ȫ���ٽ�β�����ա�
ʵ��һ��(1)�ø���������ն���������Ʒ��֤����������������ȫ�������������������β�����ӿ�˳��b��![]() д��д����
д��д����![]() ���ʴ�Ϊ��b��
���ʴ�Ϊ��b��![]() д��д����
����![]() ��
��
ʵ�����(1)���γ�һ�ܱ���ϵ�����ü������ͷ����װ�������ԣ��ʴ�Ϊ���ڵ���b���ϳ����ܣ���ĩ�˲���ˮ���У��رջ������þƾ�����Ӳ���Թ�A�����ܳ����ܿ������ݲ�������ȥ�ƾ��ƣ������γ�һ��ˮ����˵��װ�����������ã�
(2)�⻯��ʹ���������Һ��ɫ���ʴ�Ϊ���������һ��KI��Һ����Һ��ɫ��ȥ���Ұ���Ӳ��ָ�Ϊ��ɫ��
(3)Ʒ���Ǽ�����������Ƿ����Ը��������ȫ���գ�Ҳ��ʡȥƷ��װ�ã�����Ը��ݸ��������ɫ�仯��ȷ���Ƿ���ȫ���գ���B�и��������Һ����ɫ����ȥ��˵����������������ȫ���ʴ�Ϊ��������������Ƿ����Ը��������ȫ���գ���������B�и��������Һ����ɫ����ȥ��˵����������������ȫ��
(4)�������������ԭΪ�����ӣ������ӱ�����Ϊ�ⵥ�ʣ��ʴ�Ϊ��![]() ��
��
(5)��һ�εζ���������Һ��������2��3�����̫��Ӧ��ȥ��ȡ��2��3�ε�ƽ��ֵ�������Һ�����Ϊ![]() �����ݻ�ѧ����ʽ��
�����ݻ�ѧ����ʽ��![]() ֪ʣ��ĸ��������
֪ʣ��ĸ��������![]() ��������30mL��ֻȡ��3mL�����Թ�ʣ��������
��������30mL��ֻȡ��3mL�����Թ�ʣ��������![]() �����Բμӷ�Ӧ�ĸ��������
�����Բμӷ�Ӧ�ĸ��������![]() ���ٸ��ݹ�ϵʽ
���ٸ��ݹ�ϵʽ![]() �������ɶ�������
�������ɶ�������![]() ��ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�
��ȡʵ������A��Ӳ�ʲ������еIJ����������ϡ�����У���ֽ������ˣ�����Һ�м���������NaOH��Һ�����˺�ȡ�����������յ�![]() ���壬������
���壬������![]() ������
������![]() ��������Ԫ�غ���Ԫ���غ�֪
��������Ԫ�غ���Ԫ���غ�֪![]() ��
��![]() ��5��ȷ��
��5��ȷ��![]() �Ļ�ѧʽΪ��
�Ļ�ѧʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(6)a.�ζ�ʣ��![]() ��Һʱ��KI��Һ�ε���ƿ���һ�����Һƫ�࣬����ʱ������Ԫ��ƫ�٣���ֵƫ��
��Һʱ��KI��Һ�ε���ƿ���һ�����Һƫ�࣬����ʱ������Ԫ��ƫ�٣���ֵƫ��
b.����KI��Һʱ������ʱ���ӿ̶��ߣ���ʹŨ��ƫ����ʣ��������ƫС����ƫ�࣬��ֵƫС��
c.�õ⻯����Һ�ζ�ʣ��![]() ��Һʱ���ζ�ǰ�����ݣ��ζ��������ݵ��±�Һ������ƫ��ʣ��ĸ������ƫ�࣬�������Ԫ��ƫ�٣���ֵƫ��
��Һʱ���ζ�ǰ�����ݣ��ζ��������ݵ��±�Һ������ƫ��ʣ��ĸ������ƫ�࣬�������Ԫ��ƫ�٣���ֵƫ��
d.ʵ����У����������ղ���ֻ�������Ԫ��ƫ�࣬��ֵƫ��
acd��ȷ���ʴ�Ϊ��acd��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ��ش���������
(1)��֪25����NH3��H2O��Kb=1.8��105��H2SO3��Ka1=1.3��102��Ka2=6.2��108����SO2ͨ��2.0 mol��L-1��ˮ��(��Һ��������ֲ���)����c(OH)����1.0��107 mol��L1ʱ����Һ�е�![]() =______��(NH4)2SO3��Һ�е������غ�_______��
=______��(NH4)2SO3��Һ�е������غ�_______��
(2)������pHһ����4~9֮�䡣������Na2CO3�����ϸ�ʱ��pH�ɸߴ�10.5,�������ӷ���ʽ���������ʼ��Ե�ԭ��Ϊ________________(�����ӷ�Ӧ����ʽ)������ʯ��(CaSO4��2H2O)����ʹ�������Խ��ͣ��йط�Ӧ�Ļ�ѧ����ʽΪ________��
(3)SOCl2��һ��Һ̬������е�Ϊ77���������ʢ��10mLˮ����ƿ��С�ĵμ�8��10��SOCl2���ɹ۲쵽����ˮ�ⷴӦ��Һ���а����γɣ����д̼�����ζ�������ݳ����������ʹ����Ʒ����Һ����ֽ��ɫ����������ƿ���Ȱ�����ʧ������Һ�еμ�AgNO3��Һ���в�����HNO3�İ�ɫ����������
����������ʵ�飬д�� SOCl2��ˮ��Ӧ�Ļ�ѧ����ʽ________________��
��SOCl2��AlCl3��6H2O ��Ϲ��ȣ��ɵõ���ˮAlCl3����ԭ����__________��
(4)������ʧ��ʱ������ĭ���������������ʹҩҺ��ϣ������ĭ״��Ʒ����ֹ�������ӣ�����ص����ӷ�Ӧ����ʽΪ___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)��
��. [��������]
��1��Na2S2O3��5H2O����ɫ�����壬������ˮ����ϡ��Һ��BaCl2��Һ����������ɡ�
��2����Na2CO3��Na2S���Һ��ͨ��SO2���Ƶ�Na2S2O3�����ò�Ʒ�г���������Na2SO3��Na2SO4��
��3��Na2SO3�ױ�������BaSO3������ˮ��������ϡHCl��
��4�������������ⷴӦ�����ӷ���ʽΪ��2![]() +I2=
+I2=![]() +2I
+2I
��. [�Ʊ���Ʒ]ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)
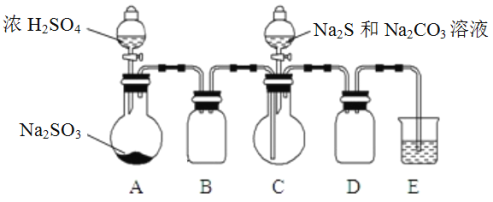
ʵ�鲽�裺
��1������ͼ��ʾ��װ��װ�ú�Ӧ��_________________________________________(���������)���ٰ�ͼʾ�����Լ�������B��D��������__________________________________________��
��2��������ƿC�м���Na2S��Na2CO3�����Һ��������ƿA�еμ�ŨH2SO4��C�з�Ӧ����Na2S2O3��CO2����ѧ����ʽΪ____________________________________________________________________��
��3����Na2S��Na2CO3��ȫ���ĺ�����Ӧ������C�л��Һ����Һ���������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
��. [̽���뷴˼]
��4����I2�ı���Һ�ⶨ��Ʒ�Ĵ��ȡ�ȡ10.0g��Ʒ�����Ƴ�100mL��Һ��������Һ������ˮ���뾭����С���ȴ�����ʹ�ã���Ŀ����ɱ������_______________________________��������̼��ȡ10.00mL��Һ����________________________________��ҺΪָʾ������Ũ��Ϊ0.10 mol/L I2�ı���Һ���еζ���������ݼ�¼���±���ʾ��
��� | 1 | 2 | 3 |
��Һ�����/mL | 10.00 | 10.00 | 10.00 |
����I2����Һ�����/mL | 19.95 | 17.10 | 20.05 |
�ζ�ʱ���ﵽ�ζ��յ��������___________________________��Na2S2O3��5H2O�ڲ�Ʒ�е�����������____________________________________(�ðٷ�����ʾ���ұ���1λС��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(BN)��һ����Ҫ�Ĺ����մɲ��ϡ�����Ȼ��ɰΪ��ʼ�����һϵ�з�Ӧ���Եõ�BF3��BN������ͼ��ʾ��
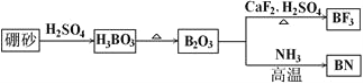
��ش��������⣺
��1����B2O3�Ʊ�BF3��BN�Ļ�ѧ����ʽ������________________________��________________________��
��2����̬Bԭ�ӵĵ����Ų�ʽΪ________��B��N��ȣ��縺�Խϴ����________��BN��BԪ�صĻ��ϼ�Ϊ________��
��3����BF3�����У�F��B��F�ļ�����________��Bԭ�ӵ��ӻ��������Ϊ________��BF3����NaF���ÿ�����NaBF4�� �����幹��Ϊ________��
��4������ʯī�ṹ���Ƶ��������������У�����Bԭ����Nԭ��֮��Ļ�ѧ��Ϊ__________�����������Ϊ________________________��
��5�������������ڸ��¸�ѹ�£�����ת��Ϊ������������ṹ����ʯ���ƣ�Ӳ������ʯ�൱�������߳�Ϊ361.5pm�������������к���________����ԭ�ӡ�________����ԭ�ӣ�������������ܶ���________g��cm-3 (ֻҪ������ʽ�����ؼ������ֵ�������ӵ�����ΪNA)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���ṹ��ʽ��ͼ��
![]()
���й��ڸ��л����˵����ȷ����( )
�ٷ���ʽΪC16H14O5��
����ʹ����KMnO4��Һ��ɫ��
���ܷ����ӳɷ�Ӧ��ȡ����Ӧ��
�ܱ����ϵ�һ�������4�֣�
��1 mol���л���ˮ��ʱ���������4 mol NaOH��
��1 mol���л�����һ�������º�H2��Ӧ��������6 mol H2��
A.�٢ڢ�B.�٢ڢۢݢ�C.�٢ܢݢ�D.�٢ڢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ����������������ԭ�����͵��ǣ� ��
A.��ˮ��������ƽ��Br2+H2O![]() HBr+HBrO��������AgNO3��Һ����Һ��ɫ��dz
HBr+HBrO��������AgNO3��Һ����Һ��ɫ��dz
B.��2HI��g��![]() H2��g��+I2��g����ƽ����ϵ����ѹǿ��ʹ��ɫ����
H2��g��+I2��g����ƽ����ϵ����ѹǿ��ʹ��ɫ����
C.��ӦCO+NO2![]() CO2+NO������ӦΪ���ȷ�Ӧ���������¶ȿ�ʹƽ�����淴Ӧ�����ƶ�
CO2+NO������ӦΪ���ȷ�Ӧ���������¶ȿ�ʹƽ�����淴Ӧ�����ƶ�
D.�ϳ�NH3��ӦΪ���ȷ�Ӧ��Ϊ���NH3�IJ��ʣ�������Ӧ��ȡ���µĴ�ʩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D�������������ͼ��ʾ��ת����ϵ����Ӧ��������Ӧ�е�ˮ����ȥ������A��B��C����ͬһ��Ԫ�ء�����˵���������
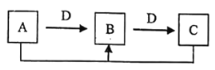
A.��A����Һ�Լ��ԣ���D�����ǵ���
B.A��C�ķ�Ӧ�����Ƿ�������ԭ��Ӧ
C.A��D������Ŀǰʹ����㷺�Ľ�������
D.��C����ʹ����ʯ��ˮ����ǵ����壬��Aһ���ǵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼװ���Ʊ�FeCO3����ȡ����FeCO3���������ᷴӦ�ɵ�����������������֪��������������(C6H11O7)2Fe�dz��õIJ�������������ˮ���ش��������⣺
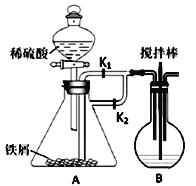
��(1)B�м���ҩƷNa2CO3��ҪʹA���Ƶõ�FeSO4��Һ����B�з�����Ӧ��ʵ�鿪ʼʱ��Ӧ��Һ©���Ļ�����______���ر�______��һ��ʱ��ر�_______����________ (�������ı��)��
(2)������������̼��������ϣ���Ӧ�Ļ�ѧ����ʽΪ(�л����÷���ʽ��ʾ)________________��
(3)�ӻ��������ĽǶȷ�������װ�ô��ڲ���֮���ǣ�_____________��
��.ij����������Ҫ�ɷ���������������������ԭ�ζ����ɲⶨ�ò���������Ԫ�صĺ�����ʵ�����Ҫ�������£�
��ȡ10Ƭ��������Ʒ���ܽ��ȥ������(�������Ԫ��)�������100mL������Һ��
����ȡ25.00mL����Һ����ƿ�С�
����c mol��L1������KMnO4��Һ�ζ����յ㣬��¼����KMnO4��Һ��������ظ�����ʵ�飬ƽ������KMnO4��Һ���ΪV mL��
(4)�õζ�ԭ�������ӷ���ʽΪ______________________
(5)����ʵ����Ӧ����ϡ�����ữKMnO4��Һ������������KMnO4��Һ�����ữ���Բⶨ�����Ӱ����________(����ƫ������ƫС��������Ӱ����)���ζ��յ��ʵ������Ϊ______��
(6)ÿƬ����������Ԫ�ص�����Ϊ__________g(�ô���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��LiBH4Ϊ�������������������о��ȵ㡣
��1����Ӧ2LiBH4=2LiH��2B��3H2��������22.4 L H2(��״��)ʱ��ת�Ƶ��ӵ����ʵ���Ϊ____mol��
��2����ͼ��2LiBH4/MgH2��ϵ�����ʱ�ʾ��ͼ����
Mg(s)��2B(s)=MgB2(s) ��H��________��

��3��������ĥ���Ʊ�Al��LiBH4�ĸ��ϲ��ϣ�����Al��LiBH4��ϵ��ˮ��Ӧ��������Խ��������о���
����ͼΪ25��ˮԡʱÿ�˲�ͬ��ȵ�Al��LiBH4���ϲ�����ˮ��Ӧ����H2�����ʱ��仯��ϵͼ����ͼ��֪������˵����ȷ����____(����ĸ)��

a��25��ʱ��������ˮ����Ӧ
b��25��ʱ����LiBH4��ˮ��Ӧ��������
c��25��ʱ��Al��LiBH4���ϲ�����LiBH4����Խ�ߣ�1000s�ڲ������������Խ��
����ͼΪ25����75��ʱ��Al��LiBH4���ϲ���[��(LiBH4)��25%]��ˮ��Ӧһ��ʱ�������X����������ͼ��(X����������������ж�ij��̬�����Ƿ���ڣ���ͬ��̬���ʳ�������������Dz�ͬ)��

��ͼ�з�����25��ʱAl��LiBH4���ϲ�������ˮ��ȫ��Ӧ��������___________(�ѧʽ)������Al(OH)3�Ļ�ѧ����ʽΪ_________________��
��4����ͼ��ֱ�����⻯�ƣ���������ȼ�ϵ��ʾ��ͼ���õ�ع���ʱ������������Һ��pH________(����������������С������������)�������ĵ缫��ӦʽΪ________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com