
ЁОЬтФПЁПЗжЮіЯТСагаЛњЛЏКЯЮяЃЌЭъГЩЬюПеЁЃ
Ђй C2H4 Ђк C2H2 Ђл ![]() Ђм
Ђм![]()
Ђн ![]() Ђо
Ђо![]() Ђп
Ђп![]() Ђр
Ђр 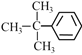 ЂсЗДЃ2ЃЖЁЯЉ
ЂсЗДЃ2ЃЖЁЯЉ
ЃЈ1ЃЉЂй~ЂсжаЃЌЪєгкБНЕФЭЌЯЕЮяЕФЪЧ____ЃЈЬюађКХЃЌЯТЭЌЃЉЃЛ
ЃЈ2ЃЉЂкЕФЕчзгЪНЮЊ___________ЃЛ
ЃЈ3ЃЉЂмЕФЯЕЭГУќУћЮЊ ___________ЃЛ
ЃЈ4ЃЉЂсЕФНсЙЙМђЪНЮЊ __________ЃЛ
ЃЈ5ЃЉЂлБЛЫсадKMnO4ШмвКбѕЛЏЕФгаЛњВњЮяЕФНсЙЙМђЪНЮЊ_____КЭ__________ЃЛ
ЃЈ6ЃЉЂрдкКЫДХЙВеёЧтЦзжага_______зщЗхЃЛ
ЃЈ7ЃЉЂйЁЂ ЂлЁЂ ЂсЕФЗаЕугЩИпЕНЕЭЕФЫГађЪЧ____________ЃЛ
ЃЈ8ЃЉаДГіЂнЕФКЌгаБНЛЗЧвгыЂнВЛЭЌРрБ№ЕФвЛжжЭЌЗжвьЙЙЬхЕФНсЙЙМђЪН________________ЁЃ
ЁОД№АИЁПЂр ![]() 2ЃЌ5-ЖўМзЛљ-2ЃЌ4-МКЖўЯЉ
2ЃЌ5-ЖўМзЛљ-2ЃЌ4-МКЖўЯЉ 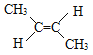
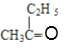 CH3COOH 4 Ђл> Ђс>Ђй
CH3COOH 4 Ђл> Ђс>Ђй 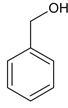 Лђ
Лђ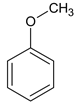
ЁОНтЮіЁП
ЃЈ1ЃЉБНЕФЭЌЯЕЮяжаКЌга1ИіБНЛЗЃЌЗжзгзщГЩЯрВюШєИЩИіCH2НсЙЙЃЛ
ЃЈ2ЃЉЂкЮЊввШВЃЌКЌгаЬМЬМШ§МќЃЛ
ЃЈ3ЃЉЂмЕФКЌгаЬМЬМЫЋМќЕФжїСДга6ИіЬМдзгЃЌЧвЮЊЖдГЦНсЙЙЃЌЫЋМќдкЕкЖўЁЂЫФИіЬМдзгЩЯЃЌМзЛљдк2ЁЂ5ИіЬМдзгЩЯЃЛ
ЃЈ4ЃЉЂсЗДЃ2ЃЖЁЯЉжа2ИіМзЛљдкЬМЬМЫЋМќЕФвьВрЃЛ
ЃЈ5ЃЉЫсадKMnO4ШмвКПЩбѕЛЏC=CМќЃЛ
ЃЈ6ЃЉЂр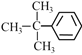 жа3ИіМзЛљЩЯЕФЧтдзгЯрЭЌЃЌБНЛЗЩЯга3жжЧтдзгЃЛ
жа3ИіМзЛљЩЯЕФЧтдзгЯрЭЌЃЌБНЛЗЩЯга3жжЧтдзгЃЛ
ЃЈ7ЃЉЂй C2H4Ђл ![]() ЂсЗДЃ2ЃЖЁЯЉЮЊЗжзгОЇЬхЃЌЗаЕуЕФИпЕЭгыЗжзгСПгаЙиЃЌЗжзгСПдНДѓЃЌЗаЕудНИпЃЛ
ЂсЗДЃ2ЃЖЁЯЉЮЊЗжзгОЇЬхЃЌЗаЕуЕФИпЕЭгыЗжзгСПгаЙиЃЌЗжзгСПдНДѓЃЌЗаЕудНИпЃЛ
ЃЈ8ЃЉЂн![]() ЕФЭЌЗжвьЙЙЬхжаКЌгаБНЛЗЃЌЧвРрБ№ВЛЭЌЃЌПЩвдЮЊДМЛђУбЃЛ
ЕФЭЌЗжвьЙЙЬхжаКЌгаБНЛЗЃЌЧвРрБ№ВЛЭЌЃЌПЩвдЮЊДМЛђУбЃЛ
ЃЈ1ЃЉБНЕФЭЌЯЕЮяжаКЌга1ИіБНЛЗЃЌЗжзгзщГЩЯрВюШєИЩИіCH2НсЙЙЃЌЮЊЂрЃЛ
ЃЈ2ЃЉЂкЮЊввШВЃЌКЌгаЬМЬМШ§МќЃЌЕчзгЪНЮЊ![]() ЃЛ
ЃЛ
ЃЈ3ЃЉЂмЕФКЌгаЬМЬМЫЋМќЕФжїСДга6ИіЬМдзгЃЌЧвЮЊЖдГЦНсЙЙЃЌЫЋМќдкЕк2ЁЂ4КХЬМдзгЩЯЃЌМзЛљдкЕк2ЁЂ5КХЬМдзгЩЯЃЌЯЕЭГУќУћЮЊ2ЃЌ5-ЖўМзЛљ-2ЃЌ4-МКЖўЯЉЃЛ
ЃЈ4ЃЉЂсЗДЃ2ЃЖЁЯЉжа2ИіМзЛљдкЬМЬМЫЋМќЕФвьВрЃЌНсЙЙМђЪНЮЊ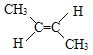 ЃЛ
ЃЛ
ЃЈ5ЃЉЫсадKMnO4ШмвКПЩбѕЛЏC=CМќЃЌВњЮяЮЊ 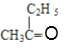 ЁЂCH3COOHЃЛ
ЁЂCH3COOHЃЛ
ЃЈ6ЃЉЂр жа3ИіМзЛљЩЯЕФЧтдзгЯрЭЌЃЌБНЛЗЩЯга3жжЧтдзгЃЌдђКЫДХЙВеёЧтЦзга4ИіЗхжЕЃЛ
жа3ИіМзЛљЩЯЕФЧтдзгЯрЭЌЃЌБНЛЗЩЯга3жжЧтдзгЃЌдђКЫДХЙВеёЧтЦзга4ИіЗхжЕЃЛ
ЃЈ7ЃЉЂй C2H4Ђл ![]() ЂсЗДЃ2ЃЖЁЯЉЮЊЗжзгОЇЬхЃЌЗаЕуЕФИпЕЭгыЯрЖдЗжзгжЪСПгаЙиЃЌЯрЖдЗжзгжЪСПдНДѓЃЌЗаЕудНИпЃЌЗаЕугЩИпЕНЕЭЕФЫГађЪЧЂл> Ђс>ЂйЃЛ
ЂсЗДЃ2ЃЖЁЯЉЮЊЗжзгОЇЬхЃЌЗаЕуЕФИпЕЭгыЯрЖдЗжзгжЪСПгаЙиЃЌЯрЖдЗжзгжЪСПдНДѓЃЌЗаЕудНИпЃЌЗаЕугЩИпЕНЕЭЕФЫГађЪЧЂл> Ђс>ЂйЃЛ
ЃЈ8ЃЉЂн![]() ЕФЭЌЗжвьЙЙЬхжаКЌгаБНЛЗЃЌЧвРрБ№гыЦфВЛЭЌЃЌЙЪЦфПЩвдЮЊДМЛђУбЃЌНсЙЙМђЪНЮЊ
ЕФЭЌЗжвьЙЙЬхжаКЌгаБНЛЗЃЌЧвРрБ№гыЦфВЛЭЌЃЌЙЪЦфПЩвдЮЊДМЛђУбЃЌНсЙЙМђЪНЮЊ![]() Лђ
Лђ![]() ЃЛ
ЃЛ


 УћЬтбЕСЗЯЕСаД№АИ
УћЬтбЕСЗЯЕСаД№АИ ЦкФЉМЏНсКХЯЕСаД№АИ
ЦкФЉМЏНсКХЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПзуСПаПгыХЈH2SO4ГфЗждкМгШШЯТЗДгІЩњГЩЛсSO2КЭH2ЕФЛьКЯЦјЬхЃЛаПКЭЯЁСђЫсЗДгІжЛгаH2ЩњГЩЁЃвбжЊЃКZn+2H2SO4(ХЈ)![]() ZnSO4+2H2O+SO2ЁќЁЃЯжгаМзввСНбаОПаЁзщЗжБ№ЪЕбщЬНОПЃК
ZnSO4+2H2O+SO2ЁќЁЃЯжгаМзввСНбаОПаЁзщЗжБ№ЪЕбщЬНОПЃК
ЃЈ1ЃЉМзбаОПаЁзщАДЯТЭМЪЕбщбщжЄаПгыХЈСђЫсЗДгІЩњГЩЮяжаSO2КЭH2ЃЌШЁЩйСПЕФZnжУгкbжаЃЌЯђaжаМгШы100mL18.5molЁЄLЃ1ЕФХЈСђЫсЃЌОЙ§вЛЖЮЪБМфЗДгІЃЌZnЭъШЋШмНтЁЃ
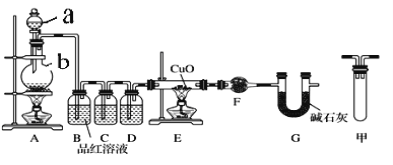
ЂйЬюаДвЧЦїУћГЦЃКa___________ЁЂb___________ЁЃ
ЂкбаОПаЁзщШЯЮЊЛЙПЩФмВњЩњЧтЦјЕФРэгЩЪЧЃК_____________________ЁЃ
ЂлзАжУDжаМгШыЕФЪдМСЪЧ__________ЁЃ
ЂмUаЭЙмGЕФзїгУЮЊ__________ЁЃ
ЂнгаЭЌбЇШЯЮЊAЁЂBМфгІдіМгЭМжаЕФМззАжУЃЌИУзАжУЕФзїгУЮЊ__________ЁЃ
ЂожЄУїЗДгІЩњГЩSO2КЭH2ЕФЪЕбщЯжЯѓЪЧ______________________________ЁЃ
ЃЈ2ЃЉввбаОПаЁзщЮЊСЫЬНОПаПгыЯЁСђЫсЗДгІЙ§ГЬжаЕФЫйТЪМАФмСПЕФБфЛЏЃЌНјаавдЯТЪЕбщЃЌЗжЮігАЯьЗДгІЫйТЪЕФвђЫиЁЃ

ЪЕбщЪБЃЌДгЖЯПЊKПЊЪМЃЌУПМфИє1ЗжжгЃЌНЛЬцЖЯПЊЛђБеКЯKЃЌВЂСЌајМЦЪ§УП1ЗжжгФкДгaЙмСїГіЕФЫЎЕЮЪ§ЃЌЕУЕНЕФЫЎЕЮЪ§ШчЯТБэЫљЪОЃК
1ЗжжгЫЎЕЮЪ§ЃЈЖЯПЊKЃЉ | 34 | 59 | 86 | 117 | Ё | 102 |
1ЗжжгЫЎЕЮЪ§ЃЈБеКЯKЃЉ | 58 | 81 | 112 | 139 | Ё | 78 |
ЗжЮіЗДгІЙ§ГЬжаЕФЫЎЕЮЪ§ЃЌЧыЛиД№ЃК
Ђй гЩЫЎЕЮЪ§58ЃО34ЁЂ81ЃО59ЃЌЫЕУїдкЗДгІГѕЦкЃЌБеКЯKЪББШЖЯПЊKЪБЕФЗДгІЫйТЪПьЃЌжївЊдвђЪЧ________ЁЃ
Ђк гЩЫЎЕЮЪ§102ЃО78ЃЌЫЕУїдкЗДгІКѓЦкЃЌЖЯПЊKЪБЕФЗДгІЫйТЪПьгкБеКЯKЪБЕФЗДгІЫйТЪЃЌжївЊдвђЪЧ______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪЕбщЙ§ГЬжаВњЩњГСЕэЕФЮяжЪЕФСП(Y)гыМгШыЪдМСЕФЮяжЪЕФСП(X)жЎМфЕФЙиЯЕе§ШЗЕФЪЧ(ЁЁЁЁ)
A. МзЯђAlCl3ШмвКжаж№ЕЮМгШыNaOHШмвКжСЙ§СПЧвБпЕЮБпеёЕД
B. ввЯђNaAlO2ШмвКжаЕЮМгЯЁбЮЫсжСЙ§СПЧвБпЕЮБпеёЕД
C. БћЯђNH4Al(SO4)2ШмвКжаж№ЕЮМгШыNaOHШмвКжБжСЙ§СП
D. ЖЁЯђNaOHЁЂBa(OH)2ЁЂNaAlO2ЕФЛьКЯШмвКжаж№НЅЭЈШыCO2жСЙ§СП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМьбщФГШмвКжаЪЧЗёКЌга![]() ЃЌГЃгУЕФЗНЗЈЪЧ( )
ЃЌГЃгУЕФЗНЗЈЪЧ( )
A.ШЁбљЃЌЕЮМгBaCl2ШмвКЃЌПДЪЧЗёгаВЛШмгкЫЎЕФАзЩЋГСЕэЩњГЩ
B.ШЁбљЃЌЕЮМгЯЁбЮЫсЫсЛЏЕФBaCl2ШмвКЃЌПДЪЧЗёгаВЛТфгкЫЎЕФАзЩЋГСЕэЩњГЩ
C.ШЁбљЃЌЕЮМгЯЁСђЫсЃЌдйЕЮМгBaCl2ШмвКЃЌПДЪЧЗёгаВЛТфгкЫЎЕФАзЩЋГСЕэЩњГЩ
D.ШЁбљЃЌЕЮМгЯЁбЮЫсЃЌЮоУїЯдЯжЯѓЃЌдйЕЮМгBaCl2ТфвКЃЌПДЪЧЗёгаВЛШмгкЫЎЕФАзЩЋГСЕэЩњГЩ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђFeCl3ШмвКжаМгШыNa2SO3ШмвК,ВтЖЈЛьКЯКѓШмвКpHЫцЛьКЯЧАШмвКжа![]() БфЛЏЕФЧњЯпШчЭМЫљЪОЁЃ
БфЛЏЕФЧњЯпШчЭМЫљЪОЁЃ
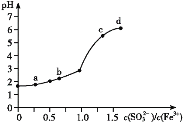
ЪЕбщЗЂЯж:
ЂЁ.aЕуШмвКГЮЧхЭИУї,ЯђЦфжаЕЮМгNaOHШмвККѓ,СЂМДВњЩњЛвАзЩЋГСЕэ,ЕЮШыKSCNШмвКЯдКьЩЋ;
ЂЂ.cЕуКЭdЕуШмвКжаВњЩњКьКжЩЋГСЕэ,ЮоЦјЬхвнГіЁЃШЁЦфЩЯВуЧхвКЕЮМгNaOHШмвККѓЮоУїЯдЯжЯѓ,ЕЮМгKSCNШмвКЯдКьЩЋЁЃ
ЯТСаЗжЮіКЯРэЕФЪЧ
A.ЯђaЕуШмвКжаЕЮМгBaCl2ШмвК,ЮоУїЯдЯжЯѓ
B.bЕуНЯaЕуШмвКpHЩ§ИпЕФжївЊдвђ:2Fe3++SO32-+H2O2Fe2++SO42-+2H+
C.cЕуШмвКжаЗЂЩњЕФжївЊЗДгІ:2Fe3++3 SO32-+6H2O![]() 2Fe(OH)3+3H2SO3
2Fe(OH)3+3H2SO3
D.ЯђdЕуЩЯВуЧхвКжаЕЮМгKSCNШмвК,ШмвКБфКь;дйЕЮМгNaOHШмвК,КьЩЋМгЩю
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯжгУNa2SO4ЙЬЬхРДХфжЦ480mL0.2molЁЄL-1ЕФNa2SO4ШмвКЁЃПЩЙЉбЁдёЕФвЧЦїШчЭМЃК
ЃЈ1ЃЉШчЭМЫљЪОЕФвЧЦїжаХфжЦШмвКВЛашвЊЕФЪЧ__ (ЬюбЁЯю)ЃЌХфжЦЩЯЪіШмвКЛЙашгУЕНЕФВЃСЇвЧЦїЪЧ___ЁЂ__ (ЬювЧЦїУћГЦ)ЁЃ
ЃЈ2ЃЉЪЙгУШнСПЦПжЎЧАБиаыНјааЕФВйзїЪЧ___ЁЃ(ЬюбЁЯю)
A.МьВщЦјУмад B.МьВщЪЧЗёТЉЫЎ C.КцИЩ
ЃЈ3ЃЉОМЦЫуЃЌашNa2SO4ЕФжЪСПЮЊ___gЁЃ
ЃЈ4ЃЉФубЁгУЕФШнСПЦПЙцИёЮЊ___mLЁЃ
ЃЈ5ЃЉХфжЦШмвКЪБЃЌвЛАуПЩЗжЮЊвдЯТМИИіВНжшЃК
ЂйГЦСП ЂкМЦЫу ЂлШмНт ЂмвЁдШ ЂнзЊвЦ ЂоЯДЕг ЂпЖЈШн
ЦфВйзїЫГађЃКЂкЁњ__Ёњ__Ёњ__Ёњ__Ёњ__Ёњ__(ЬюађКХ)ЁЃ___
ЃЈ6ЃЉдкХфжЦЙ§ГЬжаЃЌЦфЫћВйзїЖМзМШЗЃЌЖЈШнЪБИЉЪгПЬЖШЯпЛсЪЙЫљХфШмвКХЈЖШ__(ЬюЁАЦЋИпЁБЁЂЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЩшNAЮЊАЂЗќМгЕТТоГЃЪ§ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A.гЩ2HКЭ18OЫљзщГЩЕФЫЎ11 gЃЌЦфжаЫљКЌЕФжазгЪ§ЮЊ4NA
B.ГЃЮТГЃбЙЯТЕФ33ЃЎ6LТШЦјгы27gТСГфЗжЗДгІЃЌзЊвЦЕчзгЪ§ЮЊ3NA
C.0.1 mol H3O+жаКЌгаЕФЕчзгЪ§ЮЊNA
D.БъзМзДПіЯТЃЌ1LввДМЭъШЋШМЩеВњЩњCO2ЗжзгЕФЪ§ФПЮЊ![]()
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП2009Фъ10дТ1ШеЃЌЮвЙњГЩЙІОйАьЙњЧьСљЪЎФъдФБјЛюЖЏЁЃЦфжадФБјвЧЪНЩЯ9СОЕчЖЏГЕгыЛьКЯЖЏСІГЕЕШаТФмдДГЕСОЕФССЯрЃЌеЙЪОСЫзлКЯЙњСІЁЂЙњЗРПЦММЗЂеЙЫЎЦНЁЃЭЌЪБвВЫЕУїФмдДЖЬШБЪЧШЫРрЩчЛсУцСйЕФжиДѓЮЪЬтЁЃМзДМЪЧвЛжжПЩдйЩњФмдДЃЌОпгаЙуЗКЕФПЊЗЂКЭгІгУЧАОАЁЃ
ЃЈ1ЃЉЙЄвЕЩЯвЛАуВЩгУЯТСаСНжжЗДгІКЯГЩМзДМЃК
ЗДгІЂёЃК CO(g) ЃЋ 2H2(g)![]() CH3OH(g) ІЄH1
CH3OH(g) ІЄH1
ЗДгІЂђЃК CO2(g) ЃЋ 3H2(g)![]() CH3OH(g) + H2O(g) ІЄH2
CH3OH(g) + H2O(g) ІЄH2
ЂйЩЯЪіЗДгІЗћКЯЁАдзгОМУЁБддђЕФЪЧ _____ЃЈЬюЁАЂёЁБЛђЁАЂђЁБЃЉЁЃ
ЂкЯТБэЫљСаЪ§ОнЪЧЗДгІЂёдкВЛЭЌЮТЖШЯТЕФЛЏбЇЦНКтГЃЪ§ЃЈKЃЉЁЃ
ЮТЖШ | 250Ёц | 300Ёц | 350Ёц |
K | 2.041 | 0.270 | 0.012 |
гЩБэжаЪ§ОнХаЖЯІЄH1 0 ЃЈЬюЁАЃОЁБЁЂЁАЃНЁБЛђЁАЃМЁБЃЉЁЃ
ЂлФГЮТЖШЯТЃЌНЋ2 mol COКЭ6 mol H2ГфШы2LЕФУмБеШнЦїжаЃЌГфЗжЗДгІЃЌДяЕНЦНКтКѓЃЌВтЕУc(CO)ЃН 0.2 mol/LЃЌдђCOЕФзЊЛЏТЪЮЊ ЃЌДЫЪБЕФЮТЖШЮЊ ЃЈДгЩЯБэжабЁдёЃЉЁЃ
ЃЈ2ЃЉвбжЊдкГЃЮТГЃбЙЯТЃК
Ђй 2CH3OH(l) ЃЋ 3O2(g) ЃН 2CO2(g) ЃЋ 4H2O(g) ІЄH1ЃНЃ1275.6 kJ/mol
Ђк 2CO (g)+ O2(g) ЃН 2CO2(g) ІЄH2ЃНЃ566.0 kJ/mol
Ђл H2O(g) ЃН H2O(l) ІЄH3ЃНЃ44.0 kJ/mol
аДГіМзДМВЛЭъШЋШМЩеЩњГЩвЛбѕЛЏЬМКЭвКЬЌЫЎЕФШШЛЏбЇЗНГЬЪНЃК
ЃЈ3ЃЉФГЪЕбщаЁзщвРОнМзДМШМЩеЕФЗДгІдРэЃЌ
ЂйЩшМЦШчЭМЫљЪОЕФЕчГизАжУЁЃИУЕчГие§МЋЕФЕчМЋЗДгІЮЊ ЁЃ

ЂкЙЄзївЛЖЮЪБМфКѓЃЌВтЕУШмвКЕФpHМѕаЁЃЌИУЕчГизмЗДгІЕФЛЏбЇЗНГЬЪНЮЊ
ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПXЁЂYЁЂZШ§жжЮяжЪгаШчЭМЫљЪОзЊЛЏЙиЯЕЃЌЦфжаXгыЯЁбЮЫсВЛЗДгІЃК
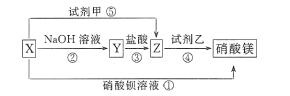
(1)ИљОнЩЯЪізЊЛЏЙиЯЕЃЌЭЦЖЯЯТСаЮяжЪЕФЛЏбЇЪНЃК X_____ЃЌY_____ЃЌZ____ЃЌЪдМСМз____ЃЌЪдМСвв____ЁЃ
(2)аДГіЩЯЪіЂйЁЋЂнВНЗДгІЕФРызгЗНГЬЪНЃК
Ђй____________________________________________ЁЃ
Ђк____________________________________________ЁЃ
Ђл____________________________________________ЁЃ
Ђм____________________________________________ЁЃ
Ђн____________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com