
����Ŀ�����ܺϽ����Բ�Ϊ�����ܶ�Ԫ�Ͻ��ڸ����£������ܿ������ܣ��������Ϊ�������������ܺϽ���Լ�ǿ�����ȶ��Խϸߣ��ͻ�ѧ��ʴ�Ժܺã���Ҫ���ں��캽���DZ��������ӱ����ſعܵȡ�
(1)��̬��ԭ�ӵļ۵����Ų�ͼΪ___________��
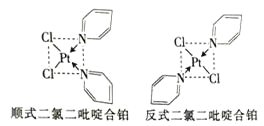
(2)���ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ�������˳ʽ�ͷ�ʽ����ͬ���칹��![]() ��ͼ
��ͼ![]() ����ѧ�о�������˳ʽ���Ӿ��п������ԡ�
����ѧ�о�������˳ʽ���Ӿ��п������ԡ�
����ष����Ǵ����ƽ�����壬�ṹ��ʽ��ͼ��ʾ����ष�����Nԭ�ӵ��ӻ���ʽ��____�������еĴ��������÷���![]() ��ʾ������m��ʾ�����γɴ�������ԭ�Ӹ�����n��ʾ�����γɴ������ĵ��Ӹ�����������еĴ�����Ӧ��ʾΪ_____��
��ʾ������m��ʾ�����γɴ�������ԭ�Ӹ�����n��ʾ�����γɴ������ĵ��Ӹ�����������еĴ�����Ӧ��ʾΪ_____��
�ڶ��ȶ���ऺϲ�������������C��N��Cl����Ԫ�صĵ�һ�������ɴ�С��˳����_____��
�۶��ȶ���ऺϲ������д��ڵ�������������_________![]() ����ĸ
����ĸ![]() ��
��
![]() ���Ӽ�
���Ӽ� ![]() ��λ��
��� ![]() ������
������ ![]() �Ǽ��Լ�
�Ǽ��Լ� ![]() ���
��� ![]() ���Լ�
���Լ�
�ܷ�ʽ���ȶ���ऺϲ�������_________![]() �������Է����������Ǽ��Է�����
�������Է����������Ǽ��Է�����![]() ��
��
�ݶ��ȶ���ऺϲ�������ƽ��ṹ��Pt2+����λ����4�����������ӻ���ʽ������sp3����������______________��
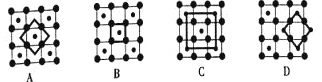
(3)�������Ͽ�ѧ����ʵ����һ������С�鷢������5K�³��ֳ����Եľ���CoO2���þ�����в�״�ṹ![]() ��ͼ��ʾ��С���ʾCoԭ�ӣ������ʾOԭ��
��ͼ��ʾ��С���ʾCoԭ�ӣ������ʾOԭ��![]() ��ͼ���ô��������ظ��ṹ��Ԫʾ��ͼ��������CoO2�Ļ�ѧ��ɵ���__________
��ͼ���ô��������ظ��ṹ��Ԫʾ��ͼ��������CoO2�Ļ�ѧ��ɵ���__________![]() ����ĸ
����ĸ![]() ��
��
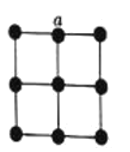
(4)�����������У���ԭ�ӵ���λ��Ϊ12��������������x��y��z���ͶӰͼ��ͼ��ʾ�������������ܶ�Ϊd g
���𰸡�![]() sp2
sp2 ![]() N>Cl>C bdf �Ǽ��Է��� ����ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ B
N>Cl>C bdf �Ǽ��Է��� ����ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ B ![]() ��
��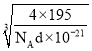
��������
(1)Co���ڵ������ڵ�VIII�壬�۵����Ų�ʽΪ3d74s2��
(2)������वĽṹ��ʽ���Կ�����Nԭ���γ�2��������N�ϻ���1�Թµ��Ӷԣ���ष�����5��̼ԭ��Ҳ��ȡsp2�ӻ���
��ͬ��������Ԫ����ԭ�����������һ�����ܳ��������ƣ�NԪ��ԭ��2p���Ϊ������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
�۶��ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ��������ڷ��Ӿ��壻
�� Cl-����ा�����Pt2+�ʶԳƽṹ��������������������غϣ�
�ݶ��ȶ���ऺϲ������У�Pt2+����λ����4�����������ӻ���ʽ������sp3��
(3)CoO2���ظ��ṹ��Ԫʾ��ͼ��Co��Oԭ����Ŀ֮��ӦΪ1:2����ͼ���֪��
A��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��2��
=1��2��
B��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��1��
=1��1��
C��Co��Oԭ����Ŀ֮��Ϊ (1+4��![]() )��4=1��2��
)��4=1��2��
D��Co��Oԭ����Ŀ֮��Ϊ4��![]() ��4��
��4��![]() =1��2��
=1��2��
(4)���ݾ����ṹ����=![]() ���㡣
���㡣
(1)Co���ڵ������ڵ�VIII�壬�۵����Ų�ʽΪ3d74s2���۵����Ų�ͼΪ��![]() ��
��
(2)������वĽṹ��ʽ���Կ�����Nԭ���γ�2��������N�ϻ���1�Թµ��Ӷԣ���ष����е�ԭ�ӵ��ӻ���ʽ��sp2�ӻ�����ष�����5��̼ԭ��Ҳ��ȡsp2�ӻ���ÿ��Cԭ�ӵ�δ�����ӻ���2p����ϵ�1�����Ӻ�Nԭ�ӵ�δ�����ӻ���2p����ϵ�1�������γɴ������������γɴ�������ԭ����Ϊ6��������Ϊ6������еĴ�������ʾΪ![]() ��
��
��ͬ��������Ԫ����ԭ�����������һ�����ܳ��������ƣ�NԪ��ԭ��2p���Ϊ������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�N��ԭ�Ӱ뾶С��Cl�ģ���2p���Ϊ������ȶ��ṹ����һ�����ܴ���Cl�ģ�Cl��̼��ȣ�ʧȥ���ӵ�������������Cl�ĵ�һ�����ܴʵ�һ������N>Cl>C��
�۶��ȶ���ऺϲ�����Pt2+��Cl-����ऽ���γɵIJ��������ڷ��Ӿ��壬����֮����ڷ��»�����Pt2+��Cl-������γ���λ����û�����Ӽ��������̼ԭ��֮���γɷǼ��Լ�����ͬԭ��֮���γɼ��Լ���C-H������ԭ�Ӳ����γ������Ҳû�н��������ʴ�Ϊ��bdf��
�ܷ�ʽ���ȶ���ऺϲ�������Cl-����ा�����Pt2+�ʶԳƽṹ��������������������غϣ����ڷǼ��Է��ӣ�
�ݶ��ȶ���ऺϲ������У�Pt2+����λ����4�����������ӻ���ʽ������sp3������������ԭ�ӹ��Ϊsp3�ӻ�����÷��ӽṹΪ�����壬��ƽ��ṹ��
(3)CoO2���ظ��ṹ��Ԫʾ��ͼ��Co��Oԭ����Ŀ֮��ӦΪ1:2����ͼ���֪��
A��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��2�����ϣ�
=1��2�����ϣ�
B��Co��Oԭ����Ŀ֮��Ϊ1��4��![]() =1��1�������ϣ�
=1��1�������ϣ�
C��Co��Oԭ����Ŀ֮��Ϊ (1+4��![]() )��4=1��2�����ϣ�
)��4=1��2�����ϣ�
D��Co��Oԭ����Ŀ֮��Ϊ4��![]() ��4��
��4��![]() =1��2�����ϣ�
=1��2�����ϣ�
��ѡB��
(4)�����������У���ԭ�ӵ���λ��Ϊ12��Ϊ���������������x��y��z���ͶӰͼ����֪��Ϊ�����������ܶѻ���Ptԭ�Ӵ��ڶ��㡢���ģ�������Ptԭ����Ŀ=8��![]() +6��
+6��![]() =4����
=4����![]() =d��a3��10-21�����
=d��a3��10-21�����![]() ��
��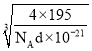 ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ȼ�ѧѭ����ͨ������ѭ��������ѭ����ȡ��������������ѭ���������Ȼ�ѧѭ����ԭ������ͼ��ʾ��

��1���������.�����ȷֽ⡱�ں����ܱ������н��У���ø����ʵ����ʵ����������¶ȵĹ�ϵ����ͼ��ʾ������650��1200��䷢������Ҫ��Ӧ�ķ���ʽΪ____��
��2���������.���������������ӷ���ʽΪ____��HI��ǿ�ᣩ��
��3�������ķ�ӦΪ2HI(g) ![]() H2(g) + I2(g) ��
H2(g) + I2(g) ��
�����ں��º����ܱ������н��и÷�Ӧ����˵���Ѵﵽƽ��״̬����___������ţ���
a���������������ѹǿ������ʱ����仯
b��n(HI)��n(H2)��n(I2)=2��1��1
c����Ӧ���ʣ�v(H2)��=v(H2)��
d��I2(g)Ũ�Ȳ�����ʱ��ı仯���仯
����֪���ѣ������ɣ�1mol��ѧ�����գ���ų�����������Ϊ���ܡ���ؼ����������£�
��ѧ�� | H��I | H��H | I��I |
����/kJ��mol��1 | 298.7 | 436.0 | 152.7 |
��÷�Ӧ��![]() HΪ____kJ��mol��1��
HΪ____kJ��mol��1��
��4����������ѭ�����в��������ͼװ�ô��漴Ϊ������ѭ������
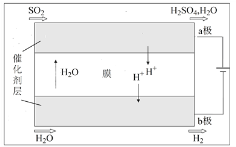
�����йظ�װ�õ����˵����ȷ����____������ţ���
a����ѧ��ת��Ϊ����
b�������ɼӿ�缫�ϵ��ӵ�ת��
c����Ӧ���ܷ���ʽΪSO2+2H2O ![]() H2+H2SO4
H2+H2SO4
d��ÿ����1molH2����·�������ĵ�����ԼΪ6.02��1023
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���й��Ŵ��Ĵ���֮һ�����ڻ�ҩ�����ı�ը��ӦΪ2KNO3��3C��S![]() A��N2����3CO2��(����ƽ)
A��N2����3CO2��(����ƽ)
(1)��S�⣬����Ԫ�صĵ縺�ԴӴ�С����Ϊ________��
(2)���������У�A�ľ�������Ϊ________�������Թ��ۼ��ķ��ӵ�����ԭ�ӹ���ӻ�����Ϊ________��
(3)��֪CN����N2�ṹ���ƣ�����HCN�����ЦҼ���м���Ŀ֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪0.1 mol��L��1�Ĵ�����Һ�д��ڵ���ƽ�⣺CH3COOH![]() CH3COO����H����Ҫʹ��Һ��
CH3COO����H����Ҫʹ��Һ��![]() ֵ�����Բ�ȡ�Ĵ�ʩ��
ֵ�����Բ�ȡ�Ĵ�ʩ��
A.�������ռ�B.�����¶�C.������������D.��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ���ǣ� ��
A. �ϳɰ���Ӧ���ȣ����õ��¿�����߰�����������
B. �����£���pH��4�Ĵ�����Һ��ˮϡ�ͣ���Һ���������ӵ�Ũ�Ⱦ�����
C. ��Ӧ4Fe(OH)2(s)��2H2O(l)��O2(g)===4Fe(OH)3(s)���������Է����У��÷�Ӧ����H<0
D. ��һ�ݻ��ɱ���ܱ������з�Ӧ2SO2(g)��O2(g)![]() 2SO3(g)��ƽ������¶Ȳ��䣬��С�����ƽ�������ƶ���
2SO3(g)��ƽ������¶Ȳ��䣬��С�����ƽ�������ƶ���![]() ��ֵ����
��ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����( )
A.��Ӧ2Mg��CO2![]() 2MgO��C ��H��0���ر�Ƕȿ������Է�����
2MgO��C ��H��0���ر�Ƕȿ������Է�����
B.���ܱ������������淴Ӧ��2NO(g)��2CO(g)![]() N2(g)��2CO2(g) ��H����113.0 kJ/mol���ﵽƽ������¶Ȳ��䣬��С������������´ﵽƽ�����H��С
N2(g)��2CO2(g) ��H����113.0 kJ/mol���ﵽƽ������¶Ȳ��䣬��С������������´ﵽƽ�����H��С
C.��֪��Ksp(AgCl)��1.8��10��10��Ksp(Ag2CrO4)��2.0��10��12���������Ũ��Ϊ1.0��10��4mol/L��AgNO3��Һ���뵽Ũ�Ⱦ�Ϊ1.0��10��4mol/L��KCl��K2CrO4�Ļ����Һ�в������ֲ�ͬ��������Ag2CrO4�����Ȳ���
D.����HClO��Ka��3.0��10��8��H2CO3��Ka1��4.3��10��7�� Ka2��5.6��10��11�����Ʋ���ͬ״���£���Ũ�ȵ�NaClO��Na2CO3��Һ�У�pHǰ��С�ں���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼʾ���Ӧ�������������

A. ͼI��ʾij���ȷ�Ӧ�ֱ����С�����������·�Ӧ�����е������仯
B. ͼ���ʾ�����£�0.1000mol��L-1 NaOH��Һ�ζ�20.00mL 0.1000mol��L-1 CH3COOH��Һ���õ��ĵζ�����
C. ͼ���ʾһ�������ı������ˮϡ�����У���Һ�ĵ��������仯���ߣ�ͼ��a��b��c�������ĵ���̶ȣ�a��b��c
D. ͼ����ʾ��Ӧ4CO(g)+2NO2(g ) ![]() N2(g)+4CO2(g) ��H ��0���������������������¸ı���ʼ��CO�����ʵ���,ƽ��ʱN2����������仯�������ͼ��֪NO2��ת����b>a>c
N2(g)+4CO2(g) ��H ��0���������������������¸ı���ʼ��CO�����ʵ���,ƽ��ʱN2����������仯�������ͼ��֪NO2��ת����b>a>c
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������־����־�����������й��ڰ�ͭ�ļ��أ���������ͭ(ͭ���Ͻ�)�������⣬����Ҫ������ң������������������Ʒ���ش��������⣺
(1)��Ԫ�ػ�̬ԭ�ӵļ۵��ӹ����ʾʽ___��3d�ܼ��ϵ�δ�ɶԵĵ�����Ϊ___��
(2)���������ڰ�ˮ�γ�[Ni(NH3)6]SO4��ɫ��Һ��
��[Ni(NH3)6]SO4�������ӵ����幹����___��
����[Ni(NH3)6]2+��Ni2+��NH3֮���γɵĻ�ѧ����Ϊ___���ṩ�µ��ӶԵijɼ�ԭ����___��
�۰��ķе�_______(������������������)�(PH3)��ԭ����______��
(3)����ͭ����������__���γɵľ��壺Ԫ��ͬ�����ĵڶ������ֱܷ�Ϊ��ICu=1959kJ/mol��INi=1753kJ/mol��ICu>INi��ԭ����___��
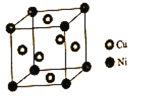
(4)ij����ͭ�Ͻ�����������ṹ��ͼ��ʾ��
�پ�����ͭԭ������ԭ�ӵ�������Ϊ___��
�����Ͻ���ܶ�Ϊdg/cm3����������a=____nm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�������ʾ��ͼһ�µ���

A��ͼ�ٱ�ʾ25��ʱ����0��1mol/L����ζ�20mL0��1mol/LNaOH��Һ����Һ��pH�����������ı仯�õ��ĵζ�����
B��ͼ�ڱ�ʾһ�������½��еķ�Ӧ2SO2(g)��O2(g)![]() 2SO3(g)��H<O���ɷֵ����ʵ����仯��t2ʱ�̸ı�����������ǽ����¶Ȼ���С�������
2SO3(g)��H<O���ɷֵ����ʵ����仯��t2ʱ�̸ı�����������ǽ����¶Ȼ���С�������
C��ͼ����ʾij������Һ�м���Ba(OH)2��Һ,���������������Ba(0H)2��Һ����Ĺ�ϵ���ڼ���20mL��Һʱ������ǡ�ó�����ȫ
D��ͼ����ʾ��ѧ��ӦH2(g)+Cl2(g)=2HCl(g)�������仯����÷�Ӧ�ķ�Ӧ�ȡ�H=+183kJ/mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com