
��Ⱦ�뻷�������Ѿ���Ϊ�����ҹ������ŵ�һ�����⣬��Ⱦ��Ϊ������Ⱦ��ˮ��Ⱦ��������Ⱦ�ȡ�
��1��Ϊ�˼��ٿ�����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϡ�
��֪��H2(g)��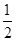 O2(g)=H2O(g) ��H1����241.8 kJ��mol��1
O2(g)=H2O(g) ��H1����241.8 kJ��mol��1
C(s)��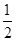 O2(g)=CO(g) ��H2����110.5 kJ��mol��1
O2(g)=CO(g) ��H2����110.5 kJ��mol��1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ�� ��
�÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ����� ��ѡ����ţ���
a��Ca(OH)2 b��CaCl2 c��Na2CO3 d��NaHSO3
��2��Ϊ�˼��ٿ����е�CO2��Ŀǰ��̼�����ڽ������������ŷ��о�����Ҫ�����ã���̼������(NH4)2CO3����ӦΪ��(NH4)2CO3(aq)��H2O(l)��CO2(g)��2NH4HCO3(aq) ��H3Ϊ�о��¶ȶ�(NH4)2CO3����CO2Ч�ʵ�Ӱ�죬��ij�¶�T1�£���һ������(NH4)2CO3��Һ�����ܱ������У�������һ������CO2���壨�õ�����Ϊϡ�ͼ�������tʱ�̣����������CO2�����Ũ�ȡ�Ȼ��ֱ����¶�ΪT2��T3��T4��T5�£�����������ʼʵ���������䣬�ظ�����ʵ�飬������ͬʱ����CO2����Ũ�ȣ����ϵ��ͼ����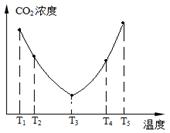
�٦�H3 0(�����������������)��
����T4��T5����¶����䣬������CO2����Ũ�ȱ仯���Ƶ�ԭ���ǣ� ��
��3�������������͵绯ѧ���ⷨ����������ˮ�������ε���Ⱦ��
�ٴ����������У���H2��NO3-��ԭΪN2��һ��ʱ�����Һ�ļ���������ǿ����Ӧ���ӷ���ʽΪ�� ��
�ڵ绯ѧ����NO3-��ԭ����ͼ����Դ����Ϊ�� ��ѡ���A����B������������ӦʽΪ�� ��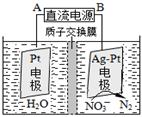
��1���� C(s)��H2O(g) �� CO(g) ��H2(g) ��H����131.3kJ��mol��1 k��c CO)��c( H2)/c(H2O)
�� a c����1�֣�
��2���� <
�� T4��T5 ��Ӧ��ƽ�⣬����ӦΪ���ȷ�Ӧ�������¶ȵ����ߣ�ƽ�������ƶ���CO2������Ч�ʽ��ͣ���NH4HCO3���ַֽ⣬��˼��������֣���
��3����2 NO3����5H2 N2��2OH����4H2O
N2��2OH����4H2O
��A 2 NO3����12 H+��10e��= N2����6H2O
���������������1���������������Ȼ�ѧ����ʽ��ʽ����ʽ����C(s)��H2O(g) �� CO(g) ��H2(g) ��H����131.3kJ��mol��1�����Լ�Ca(OH)2��Na2CO3���������Ӧ��������Ϊ�����ռ�����2���ٸ�ͼ����ͬʱ��ʱ�ⶨ�Ķ�����̼��Ũ�ȣ��¶�Խ�߷�Ӧ����Խ�죬�ȴ�ƽ�⣬����ͼ���T3Ϊ�磬������ƽ�����ߣ��ݴ˷����¶ȸ߶�����̼������ƽ�������ƶ�������ӦΪ���ȷ�Ӧ����H3< 0����T4��T5 ��Ӧ��ƽ�⣬����ӦΪ���ȷ�Ӧ�������¶ȵ����ߣ�ƽ�������ƶ���CO2������Ч�ʽ��ͣ���3���÷�Ӧ�������ϼ����ߣ�������Һ������ǿ����������OH�����ɴ���д����ʽ��������B�����ĵ缫NO3���õ���������N2��Ϊ��������AΪ������BΪ������
���㣺���黯ѧԭ���ۺ����ݡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�ڱ���¯�з�����Ӧ��
��Fe2O3(s)��3C(s)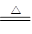 2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
2Fe(s)��3CO(g)����H����492.7 kJ��mol��1
��3CO(g)��Fe2O3(s)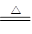 2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
2Fe(s)��3CO2(g)����H����25.2 kJ��mol��1
��Ӧ2Fe2O3(s)��3C(s)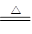 4Fe(s)��3CO2(g)����H��________kJ��mol��1��
4Fe(s)��3CO2(g)����H��________kJ��mol��1��
(2)��Ȼ��(�Լ����)�ڹ�ҵ��������;�㷺����������ת������H2����Ҫת����Ӧ���£�
CH4 (g)��H2O(g) CO(g)��3H2(g)����H����206.2 kJ��mol��1
CO(g)��3H2(g)����H����206.2 kJ��mol��1
CH4(g)��2H2O(g) CO2(g)��4H2(g)����H����165.0 kJ��mol��1
CO2(g)��4H2(g)����H����165.0 kJ��mol��1
������Ӧ����ԭ�����е�CO��ʹ���ϳɴ����ж��������ȥ����ҵ�ϳ����ô���������CO��ˮ������Ӧ�����׳�ȥ��CO2��ͬʱ�ֿ��Ƶõ�����������ķ������˷�Ӧ��Ϊһ����̼�任��Ӧ���÷�Ӧ���Ȼ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��һֱ�����ڡ��˹��̵����ķ����о���
(1)�ϳɰ���ԭ��Ϊ��N2(g)+3H2(g) 2NH3(g)��H="-92.4" kJ/mol���÷�Ӧ�������仯��ͼ��ʾ��
2NH3(g)��H="-92.4" kJ/mol���÷�Ӧ�������仯��ͼ��ʾ��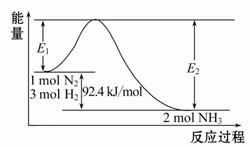
���ڷ�Ӧ��ϵ�м����������Ӧ��������E2�ı仯�� (���������С�����䡱)��
�ڽ�0.3 mol N2��0.5 mol H2�������������ܱ������У���һ�������´ﵽƽ�⣬�������������ѹǿ��Ϊԭ����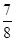 ����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
����ʱH2��ת����Ϊ ������߸�������H2��ת���ʣ����д�ʩ���е��� (��ѡ����ĸ)��
| A���������а�ԭ�����ٳ���ԭ���� |
| B�����������ٳ���һ����H2 |
| C���ı䷴Ӧ�Ĵ��� |
| D��Һ�������������� |
 4NH3(g)+3O2(g)
4NH3(g)+3O2(g) H2O(g) ��H="+44.0" kJ/mol
H2O(g) ��H="+44.0" kJ/mol 4NH3(g)+3O2(g) ��H= kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)��
4NH3(g)+3O2(g) ��H= kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK �����������������䣬����ѹǿ��Kֵ (���������С�����䡱)���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ���ʼ��仯�����ڹ�ҵ�����Ϳ���������Ҫ���á�
��1����֪��2Cu2O(s) + O2(g) = 4CuO(s)��H����292kJ��mol��1
2C(s)+O2(g)=2CO(g) ��H����221kJ��mol��1
��д��������̿�ۻ�ԭCuO��s���Ʊ�Cu2O��s�����Ȼ�ѧ����ʽ�� ��
��2�������Ȼ�ͭ����(CuCl2��2H2O�����Ȼ���������)��ȡ������CuCl2��2H2O���Ƚ����Ƴ�ˮ��Һ������ͼ��������ᴿ: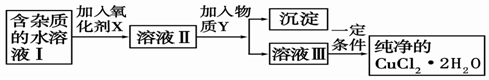
��֪Cu2+��Fe3+��Fe2+���������↑ʼ�����ͳ�����ȫʱ��pH���±�
| �������� | Fe3+ | Fe2+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1�� ��һ���¶��£����0.1 mol��L��1CH3COOH��Һ��PHΪ3.0����CH3COOH��ˮ�еĵ���Ϊ �����¶�CH3COOH�ĵ���ƽ�ⳣ��Ϊ ��
��2�� ��25��ʱ��Kw��1.0��10��14�����0.1 mol��L��1 Na2A��Һ��pH��7����H2A��ˮ��Һ�еĵ��뷽��ʽΪ �����¶��£���0.01 mol��L��1 H2A��Һϡ�͵�20������Һ��pH�� ��
��3�� ��֪HCN(aq)+NaOH(aq)��NaCN(aq)+ H2O(l) ��H����12.1 kJ��mol��1��
HCl(aq) +NaOH(aq)��NaCl(aq) + H2O(l) ��H����57.3 kJ��mol��1��
����ˮ��Һ��HCN H++CN������Ħ�HΪ kJ��mol��1
H++CN������Ħ�HΪ kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����Ź㷺����;�������ڻ��ʡ����ᡢ�ϳ���ά�ȹ�ҵ������
��1���������¡��˹��̵������о��������ڳ��¡���ѹ�����������£�N2�ڴ�������������Fe2O3��TiO2��������ˮ������Ӧ�����ɰ�����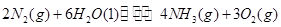
�÷�Ӧ�ڹ̶�������ܱ������н��У��й�˵����ȷ����_____________���������ĸ����
A����Ӧ����ƽ��״̬ʱ��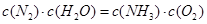 |
B����Ӧ�ﵽƽ���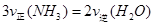 |
| C����ϵ����ѹǿ���䣬˵����Ӧ�Ѵ�ƽ�� |
| D�����������ܶȱ��ֲ��䣬˵����Ӧ�Ѵ�ƽ�� |
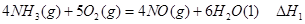 ��
��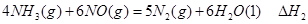 ��
��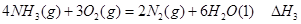 ��
��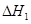 ��
��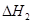 ��
��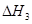 ����֮���ϵ�ı���ʽ��
����֮���ϵ�ı���ʽ��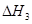 ��_________��
��_________��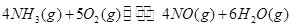
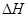 ��
��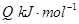


| ʱ��/Ũ�� |  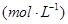 | 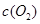 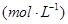 | 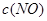 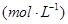 | 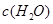 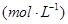 |
| ��ʼ | 4.0 | 5.5 | 0 | 0 |
| ��2min | 3.2 | a | 0.8 | 1.2 |
| ��4min | 2.0 | 3.0 | 2.0 | 3.0 |
| ��6min | 2.0 | 3.0 | 2.0 | 3.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ��Ӧ�м������ʱ仯�����������仯���ͷŻ����������ǻ�ѧ��Ӧ�������仯����Ҫ��ʽ֮һ����֪C(ʯī)��H2(g)ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
�� C(ʯī)+ O2(g)��CO(g)
O2(g)��CO(g) 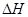 ="-111.0" KJ��mol-1
="-111.0" KJ��mol-1
�� H2(g)+  O2(g) ��H20(g)
O2(g) ��H20(g) 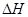 ="-242.0" kJ��mol-1
="-242.0" kJ��mol-1
�� C(ʯī)+O2(g)��CO2(g) 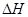 ="-394.0" kJ��mol-1
="-394.0" kJ��mol-1
�����������⣺
��1����ѧ��Ӧ���������仯�ı���ԭ���Ƿ�Ӧ�������� �Ķ��Ѻ��γɡ�����������Ӧ���� (����ȡ����ȡ�)��Ӧ��
��2�����Ȼ�ѧ����ʽ�У���Ҫ������Ӧ�P�������״̬��ԭ���� ���ڢ��У�02�Ļ�ѧ��������1/2���DZ�ʾ (����ĸ)��
a�����Ӹ��� b�����ʵ��� c����������
��3����Ӧ2H20(g)��2H2(g)+02(g)��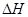 = KJ��mol-1��
= KJ��mol-1��
��4����C(���ʯ)+02(g)��C02(g)��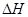 ="-395.0" kJ��mol-1�����ȶ��ԣ����ʯ (�����������������)ʯī��
="-395.0" kJ��mol-1�����ȶ��ԣ����ʯ (�����������������)ʯī��
��5����֪�γ�H20(g)�е�2 mol H-O���ܷų�926.0 kJ���������γ�1 mol 02(g)�еĹ��ۼ��ܷų�498.0 kJ�������������1 mol H2(g)�е�H-H����Ҫ������ KJ��
��6����ҵ��������һ����Ҫ;������CO(g)��H2O(g)��Ӧ����C02(g)��H2(g)����÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������һЩ���ʵ��۷е����ݣ���ѹ����
| | �� | �� | Na2CO3 | ���ʯ | ʯī |
| �۵㣨�棩 | 63��65 | 97��8 | 851 | 3550 | 3850 |
| �е㣨�棩 | 774 | 882��9 | 1850���ֽ����CO2�� | ---- | 4250 |
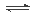 2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol
2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol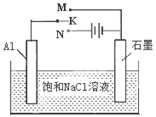
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ѣ�CH3OCH3����һ����Ҫ�ľ�ϸ������Ʒ������Ϊ�Ƕ�ʮһ��������DZ����ȼ��[ ��֪��CH3OCH3(g)+3O2(g)��2CO2(g)+3H2O(1) ��H����1455kJ/mol ]��ͬʱ��Ҳ������Ϊ�������������ȴ�������ҵ���Ʊ������ѵ���Ҫ���������������Σ�
�ټ״�Һ����Ũ���������»�״������ڴ�������ֱ����ˮ�ƶ����ѣ�2CH3OH CH3OCH3��H2O
CH3OCH3��H2O
�ںϳ���CO��H2ֱ�Ӻϳɶ����ѣ�3H2(g)��3CO(g) CH3OCH3(g)��CO2(g) ��H����247kJ/mol
CH3OCH3(g)��CO2(g) ��H����247kJ/mol
����Ȼ����ˮ������Ӧ�Ʊ������ѡ���CH4��H2OΪԭ���Ʊ������Ѻͼ״���ҵ�������£�
��1��д��CO(g)��H2(g)��O2(g)��Ӧ����CO2(g)��H2O(1)���Ȼ�ѧ����ʽ���������һλС���� ��
��2���ٷ������ü״�Һ����Ũ��������ֱ����ˮ�ƶ����ѣ����ܲ��ʸߣ���������̭����Ҫԭ���� ��
��3���ڷ�Ӧ��2�У�һ�������·�����Ӧ3H2(g)��3CO(g) CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
CH3OCH3(g)��CO2(g)���ܱ������дﵽƽ���Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ��
A�����¸�ѹ B���Ӵ��� C������COŨ�� D�������������
��4���ڷ�Ӧ��3�У���һ���¶Ⱥ�ѹǿ�����·����˷�Ӧ��3H2(g)��CO2(g)  CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ���
CH3OH(g)��H2O (g) ��H��0��Ӧ�ﵽƽ��ʱ���ı��¶ȣ�T����ѹǿ��P������Ӧ�����CH3OH�����ʵ����������仯�����ͼ��ʾ�������¶ȣ�T����ѹǿ��P���Ĺ�ϵ�ж���ȷ���� ������ţ���
A��P3��P2 T3��T2 B��P2��P4 T4��T2
C��P1��P3 T1��T3 D��P1��P4 T2��T3
��5����Ӧ��1�з�����Ӧ��CH4(g)��H2O(g) CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ�� ������¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ��������䡱�����������С����
CO(g)��3H2(g) ��H��0д��ƽ�ⳣ���ı���ʽ�� ������¶Ƚ��ͣ��÷�Ӧ��ƽ�ⳣ�� ��������䡱�����������С����
��6����ͼΪ��ɫ��Դ��������ȼ�ϵ�ء��Ĺ���ԭ��ʾ��ͼ����a�缫�ķ�ӦʽΪ��________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com