
����Ŀ������������Ԫ��A��B��C��D��ԭ������������������A��Cͬ���壬B��C��Dͬ���ڣ�Aԭ�ӵ������������Ǵ�����������3����B�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�ء��Իش��������⣺
��1��A��Ԫ�ط���___________��B��Ԫ������___________��
��2��D��ԭ�ӵĵ���ʽ____________��Cԭ�ӵĵ����Ų�ʽ___________________��
��3��A��B��C����Ԫ���γɵļ����ӵİ뾶�ɴ�С��˳����_____________��
��4��CA2��DԪ�صĵ�����ˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��_______________________��
���𰸡� O �� �� 1s22s22p6 3s23p4 S2-��O2-��Na+ SO2 + Cl2 + 2H2O == H2SO4 + 2HCl
���������������������������Ԫ��A��B��C��D��ԭ��������������Aԭ�ӵ������������Ǵ�����������3������AΪOԪ�أ�A��Cͬ���壬CΪSԪ��,DΪClԪ�أ�B��C��Dͬ���ڣ�B�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�أ���BΪNaԪ�أ���D��ԭ�ӵĵ���ʽΪ��![]() ��Cԭ�ӵĵ����Ų�ʽ1s22s22p63s23p4��A��B��C����Ԫ���γɵļ�����O2��Na+��S2-����������S2-���Ӳ�����࣬��S2-�뾶���O2��Na+���Ӳ�����ͬ����O2-�ĺ˵����С����뾶���ʰ뾶�ɴ�С˳��Ϊ��S2->O2->Na+��CA2��DԪ�صĵ�����ˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��SO2 + Cl2 + 2H2O == H2SO4 + 2HCl��
��Cԭ�ӵĵ����Ų�ʽ1s22s22p63s23p4��A��B��C����Ԫ���γɵļ�����O2��Na+��S2-����������S2-���Ӳ�����࣬��S2-�뾶���O2��Na+���Ӳ�����ͬ����O2-�ĺ˵����С����뾶���ʰ뾶�ɴ�С˳��Ϊ��S2->O2->Na+��CA2��DԪ�صĵ�����ˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��SO2 + Cl2 + 2H2O == H2SO4 + 2HCl��


 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.��ѧ��һ����ʵ��Ϊ������ѧ�ƣ���ѧ��ȡ�õķ�˶�ɹ���ʵ�����Ҫ���÷ֲ������������ʵ��װ��ͼ�ش����⣺

��1��д������ͼ�����������ƣ���_______����____��
��2��������װ��I�������ᣨ�е�118�����������������е�77.1�����Ļ�����ȱ�ٵIJ���������________��������������������е�ʵ�����������Ϊ_______��ʵ��ʱ����������ȴˮ�Ľ���Ϊ____������f���� ��g������������ƿ�����Ƭ��������________��
��3����������250 mL 0.1 moI.L-1NaCl��Һ��װ��II��ijͬѧת����Һ��ʾ��ͼ��ͼ������������ֱ���________��________��
II. �ٺ������������ʾ����������Եõ���ˮ�����ˮ�м������Ȼ�̼����ȡ�ⵥ�ʵ�ʵ�������________���ò�����Ҫ�IJ���������________��
��ijNaCl��Ʒ�п��ܺ���SO42-��CO32-��Ϊ�����������ӵĴ��ڣ���ȡ����ʵ�鲽�裺
��Ʒ![]() ����������
����������![]() ����������
����������
�������Լ�AΪ_______��BΪ_______��������֤����Ʒ�в�����____��
���ں���ʵ������Ҫʹ��450mL0.5 mol��L- 1NaCl��Һ��Ϊ���Ƹ�Ũ��NaCl��Һ����ʵ�飬����������ƽ��ȡNaCl_______g������NaCl��Һʱ�����������в�������ʹ����Ũ��ƫ�ߵ���____________
A����ƽ���뼺��ʴ B�����ƹ�����δ������ˮϴ���ձ��Ͳ�����
C��ת����Һʱ����Һ���� D������ʱ���ӿ̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)98%��ŨH2SO4(��=1.84g/ mL)��Ũ��Ϊ______mol/L���ø�Ũ��������500ml0.5mol/L��ϡH2SO4����Ҫ��ȡŨH2SO4�����Ϊ_______mL(С�������һλ��Ч����)��
(2)���ʵ������10mL��20mL��50mL��Ͳ��Ӧѡ��______mL��Ͳ��ʵ���л���Ҫ�õ��IJ����������ձ�������������ͷ�ιܡ�_________________________��
(3)ʹ������ƿǰ������е�һ��������_____________________��
(4)����ʱ,һ���Ϊ���¼�������:
����ȡ �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ��ҡ��
����ȷ�IJ���˳��Ϊ________________________��
A. �ڢ٢ۢ�ݢ�ߢޢ� B. �ڢ٢ۢ�ݢޢ�ߢ� C. �ڢ٢ۢݢޢߢܢ��
(5) ��ʵ���г��������������������ҺŨ��ƫ�ߵ���______
A.Ũ����ϡ�ͺ�δ�������¼�ת��������ƿ���ж���
B.����ʱ���ӿ̶���
C.��ȡ��Ũ���ᵹ���ձ��ܽ����ˮϴ����Ͳ2-3�Σ���ϴ��Һ�����ձ�
D����Һ���ձ�δϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и���ָ����Ԫ�أ������γ�AB2�ͻ�������ǣ� ��
A.2s22p2 ��2s22p4 B. 3s23p4 ��2s22p4 C.3s2��2s22p5 D. 3s1��3s23p5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����(����)
A. Ǧ���طŵ�ʱ�����������������ʱ������������
B. ͬ������0.1 mol��L��1������ҺpH��a��0.01 mol��L��1������ҺpH��b����a��1<b
C. ����ˮբ����������������������������ӵ������������������з���
D. һ�������·�ӦN2��3H2![]() 2NH3����3v��(H2)��2v��(NH3)����Ӧ�ﵽƽ��
2NH3����3v��(H2)��2v��(NH3)����Ӧ�ﵽƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳ��÷�¯��(��Ҫ��FeO��V2O5����������SiO2��P2O5������)��ȡV2O5���������£�

��1�����յ�Ŀ���ǽ�FeO��V2O3ת��Ϊ������NaVO3���ù����б�������Ԫ����_______________������������Ҫ�ɷ�Ϊ____________________(�ѧʽ)��
��2����MgSO4��Һ���衢��ʱ����������Ҫ�ɷ�Ϊ__________��
��3���ڱ���NH4VO3�Ĺ����У����������ļ���ֵ(������)���¶ȱ仯��������ͼ��ʾ��210��ʱ��ʣ��������ʵĻ�ѧʽΪ_____________________��

��4����V2O5ұ���������������ȷ����������ȷ�Ӧ��ʵ�������__________________.
��5����V2O5��������ϡ����õ�250mL(VO2)2SO4��Һ��ȡ25.00mL����Һ����ƿ�У���0.1000 mol��L-1H2C2O4����Һ���еζ����ﵽ�ζ��յ�ʱ���ı���Һ�����Ϊ20.00mL����֪�ζ�������H2C2O4������ΪCO2��VO2+(��ɫ)����ԭΪVO2+(��ɫ)��
�ٸõζ�ʵ�鲻��Ҫ�������ָʾ�����ﵽ�ζ��յ��������___________________��
��(VO2)2SO4��Һ�����ʵ����ʵ���Ũ��Ϊ___________________��
�۴ﵽ�ζ��յ�ʱ�����ӵζ��ܶ�����ʹ���_________(����ƫ��������ƫ����������Ӱ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ȲΪԭ�ϣ���ȡCH2Br-CHBrCl�����еķ�Ӧ;���ǣ� ��
A.�ȼ�Cl2���ټ�Br2B.�ȼ�Cl2���ټ�HBr
C.�ȼ�HCl���ټ�HBrD.�ȼ�HCl���ټ�Br2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������л������������ԭ������������ȷ����
A. 3-��-2-��ϩ B. 2,2-��������
C. 3-�ȶ��� D. 2-�һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(Ce)����ϵ����Ԫ�ء�������Ⱦ��NOͨ���ú�Ce4+����Һ���գ�����HNO2��NO3���������õ�ⷨ����������Һ�е�HNO2ת��Ϊ�����ʣ�ͬʱ����Ce4+����ԭ����ͼ��ʾ������˵����ȷ����
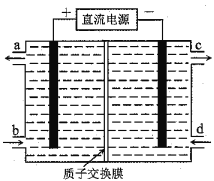
A. H+�����ҽ�������
B. Ce4+�ӵ��۵�c���������ҿ�ѭ��ʹ��
C. �����ĵ缫��Ӧʽ��2HNO2+6H++6e��=N2��+4H2O
D. ���ü���ȼ�ϵ����Ϊ��Դ�������ı�״����33.6L����ʱ�������Ͽ�ת��HNO22mol
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com