
��0.02 mol Na��Ͷ�뵽ʢ��100 mLˮ��100 mL 1mol ���ᡢ100 mL 1mol
���ᡢ100 mL 1mol ����ͭ��Һ��X��Y��Z�����ձ��У������й�˵���������( )��
����ͭ��Һ��X��Y��Z�����ձ��У������й�˵���������( )��
A�������ձ���һ�����ᷢ�������ӷ�Ӧ�У�2Na+2H2O==2Na++2OH��+H2��
B�������ձ����ƾ���Һ���Ͼ��ҷ�Ӧ����ȶ��ԣ�X�ձ��еķ�Ӧƽ��Щ
C��Z�ձ���һ�����г������ɣ����������ǵ���ͭ
D�������ձ�����������������ʵ�����ͬ
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�⻯�ƹ����ǵ�ɽ�˶�Ա���õ���Դ�ṩ����ij��ȤС����ѡ������ͼ��ʾװ���Ʊ��⻯�ơ�

��ش��������⣺
(1)��ѡ���Ҫ��װ�ã���������������˳��Ϊ________________________________________________________________________
(�������ӿڵ���ĸ���)��
(2)����������ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©��������________(�밴��ȷ��˳���������в���ı��)��
A�����ȷ�Ӧһ��ʱ��
B���ռ����岢�����䴿��
C���رշ�Һ©������
D��ֹͣ���ȣ������ȴ
(3)ʵ�������ijͬѧȡ�������С�ļ���ˮ�У��۲쵽������ð������Һ�м����̪���Ժ�ɫ����ͬѧ�ݴ��жϣ�����ʵ��ȷ��CaH2���ɡ�
��д��CaH2��ˮ��Ӧ�Ļ�ѧ����ʽ��__________
_____________________________________________��
�ڸ�ͬѧ���жϲ�ȷ��ԭ���ǣ�____________
___________________________________________��
(4)�������һ��ʵ�飬�û�ѧ�������ָ����⻯�ƣ�д��ʵ���Ҫ���輰�۲쵽������______________________��
(5)��ɽ�˶�Ա�����⻯����Ϊ��Դ�ṩ������������ȣ����ŵ���________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ȩ��ʳƷ��ҽҩ�������ȷ��涼��Ӧ�á����������dz����ڵ��ƾ��в�ݮ �����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��
�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫��
��1�����ȩ��C��H��O����Ԫ����ɣ����������ȩ���ӵ���Է�������Ϊ132���������̼Ԫ�ص���������Ϊ81��8%�����ȩ�ķ���ʽ�� _______________�����ȩ�DZ���һȡ����˴Ź���������ʾ�����������������ֲ�ͬ��ѧ��������ԭ�ӣ���ṹ��ʽ��________________________����������˳���칹���ӳ�칹��
��2����֪��
I��ȩ��ȩ�ܷ�����Ӧ��ԭ�����£�

II���ϳ����ȩ�Ĺ�ҵ��������ͼ��ʾ�����м�Ϊij������

��д�����Ͷ��������Ļ�ѧ����ʽ��__________________________________��
��3�����ȩ�ܱ�������Һ�������پ��� ���õ�����ᣬд�����������Ľṹ��ʽ_________________________________________________________��
���õ�����ᣬд�����������Ľṹ��ʽ_________________________________________________________��
��4�����÷�����A Ϊԭ�Ϻϳ���������H��·�����£����A�ĺ˴Ź���������ͼ��6���壬�����֮��Ϊ1�U2�U2�U2�U1�U2��
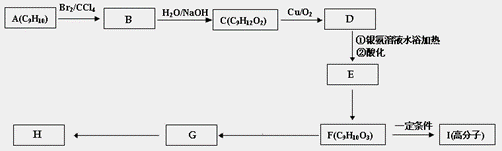
�ٷ�����A�Ľṹ��ʽ_________________________________________________��
�ڻ�����F�еĹ�������_______________________________________�������ƣ���
��G��H�Ļ�ѧ����ʽ��________________________________________________��
��G��ͬ���칹���У���������Ŀ������ֻ��һ��ȡ������ͬ���칹����____�֡���������һ�ֵĽṹ��ʽ��_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ʵ������ƽ���ƶ�ԭ�����͵���
|
|
|
| |||||||||||||
| A | B | C | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
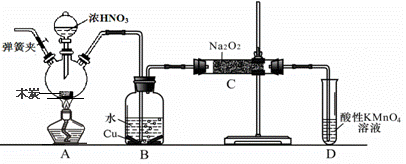
�������Ʊ���Ϊ��ҵ�Σ���Ư�ס���Ƶȷ���Ӧ�ù㷺����ľ̿��Ũ���ᡢˮ��ͭΪԭ�����ɵ�һ��������������Ʒ�Ӧ�Ʊ��������Ƶ�װ������ͼ��ʾ�����ּг�װ���ԣ���
��֪�������£���2NO+Na2O2 == 2NaNO2
��3NaNO2+3HCl == 3NaCl+HNO3+2NO��+H2O��
�����������£�NO��NO2�C ������MnO4�C��Ӧ����NO3�C ��Mn2+
��������������
���������գ�
��1��д��Ũ������ľ̿��Ӧ�Ļ�ѧ����ʽ ��
��2��B�й۲쵽����Ҫ����������ɫ��������� ��Dװ�õ������� ��
��3������C�в������������Ƶķ����� ��
��4��������C�����г�������������и�����̼���ƺ��������ƣ�Ϊ���������Щ������Ӧ��B��Cװ�ü�����װ�ã����װ����ʢ�ŵ�ҩƷ���� ��
��5����1.56g����������ȫת����Ϊ�������ƣ�������������Ҫľ̿ g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
NA���������ӵ�����������������ȷ����
A��1 mol Cl2�ڷ�Ӧ�еõ���������һ��Ϊ2NA
B��1 mol O2�ڷ�Ӧ�еõ�������һ��Ϊ4 NA
C��1 mo1 Na2O2������H2O��Ӧ��ת����2 NA ������
D����2KClO3+4HC1(Ũ)= =2KCl+2C1O2��+C12��+2H2O�У��������������������ǻ�ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʵ������ͬһԭ�����͵���
A��Ũ�������ˮ����ɫ�Լ�ƿ����
B�����ƺ��������ƹ��峤�ڱ�¶�ڿ����б���
C�����������Ͳ���������Ũ����
D��SO2��Na2SO3��Һ����ʹ��ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڷ�ӦaBrF3 �� bH2O �� cHBrO3 �� dHBr �� eHF �� fO2����(a��b��c��d��e��f
�Ǹ����ʵĻ�ѧ������)����0.3molH2O����������ˮ��ԭ��BrF3�����ʵ�����
A��0.15mol B��0.2mol C��0.3mol D��0.4mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ϳɰ��������ѧ�����ϵ�һ���ش�ͻ�ƣ��䷴Ӧԭ��Ϊ
N2(g)��3H2(g) 2NH3(g)����H����92.4 kJ��mol��1��
2NH3(g)����H����92.4 kJ��mol��1��
һ�ֹ�ҵ�ϳɰ��ļ�ʽ����ͼ���£�

(1)��Ȼ���е�H2S���ʳ��ð�ˮ���գ�����ΪNH4HS��һ����������NH4HS��Һ��ͨ��������õ�������ʹ����Һ������д��������Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(2)���������������ԭ�����£�
��CH4(g)��H2O(g)  CO(g)��3H2(g)
CO(g)��3H2(g)
��H����206.4 kJ��mol��1
��CO(g)��H2O(g)  CO2(g)��H2(g)
CO2(g)��H2(g)
��H����41.2 kJ��mol��1
���ڷ�Ӧ�٣�һ���������ƽ����ϵ��H2�İٷֺ��������ܼӿ췴Ӧ���ʵĴ�ʩ��____________��
a�������¶ȡ�b������ˮ����Ũ�ȡ�c�����������d������ѹǿ
���÷�Ӧ�ڣ���CO��һ��ת���������H2�IJ�������1 mol CO��H2�Ļ������(CO���������Ϊ20%)��H2O��Ӧ���õ�1.18 mol CO��CO2��H2�Ļ�����壬��CO��ת����Ϊ____________��
(3)ͼ(a)��ʾ500 �桢60.0 MPa�����£�ԭ����Ͷ�ϱ���ƽ��ʱNH3��������Ĺ�ϵ������ͼ��a�����ݼ���N2��ƽ�����������____________��
(4)�����¶ȶԺϳɰ���Ӧ��Ӱ�죬��ͼ(b)����ϵ�У�����һ�������µ��ܱ������ڣ���ͨ��ԭ������ʼ�����¶Ȳ������ߣ�NH3���ʵ����仯������ʾ��ͼ��
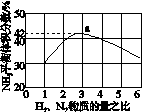 ��
��
(a)������������������(b)
(5)��������ͼ�У�ʹ�ϳɰ��ų��������õ�������õ���Ҫ������(�����)________����������������ߺϳɰ�ԭ����ת���ʵķ�����________________________________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com