
����Ŀ����ɫ��ѧ�Ի�ѧ��Ӧ�������ԭ�Ӿ�������(ԭ�ӽ�Լ)���¸��Ҫ�������ԭ�Ӿ����Է�Ӧ��ԭ�Ϸ����е�ԭ��ȫ��ת��������������������ʵ�����ŷš����м��������ұ��ķ����У�ԭ�Ӿ�������õ���(��Ӧ����һ�������½���)( )
A.![]() +C2H5Cl��
+C2H5Cl��![]() +HCl
+HCl
B.![]() +C2H5OH��
+C2H5OH��![]() +H2O
+H2O
C.![]() +CH2=CH2��
+CH2=CH2��![]()
D.![]() ��
��![]() +HBr��
+HBr��![]() +H2��
+H2��![]()
 ������ϵ�д�
������ϵ�д� �±�Сѧ��Ԫ�Բ���ϵ�д�
�±�Сѧ��Ԫ�Բ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25��C���ı�0.01 mol��Lһ1 CH3COONa��Һ��pH.��Һ��c��CH3COOH����c��CH3COO-����c��H +����c��OH- ���Ķ���ֵlgc����ҺpH�ı仯��ϵ��ͼ��ʾ������������ȷ����
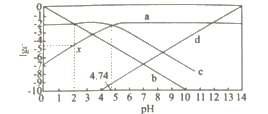
A.ͼ������������c��CH3COOH��+c��CH3COO-��=c��Na+��
B.0.01 mol��L-1CH3COOH��pHԼ������a����c���㴦�ĺ�����ֵ
C.��ͼ����Ϣ�ɵõ�x��������ֵΪ4.74
D.25��Cʱ��![]() ��ֵ�� pH�����������
��ֵ�� pH�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ�����������Ҫ�������£�
������һ��������Ũ������Ũ����Ļ���ᣬ���뷴Ӧ���С�
���������µĻ��������μ���һ�����ı����������Ͼ��ȡ�
����50��60���·�����Ӧ��ֱ����Ӧ������
�ܳ�ȥ�����ֲ�Ʒ������H2O��5%NaOH��Һϴ�ӣ��������H2Oϴ�ӡ�
�ݽ���ˮCaCl2�����Ĵ��������������õ�����������
(1)����һ������Ũ������Ũ��������ʱ������ע��������_____________________________________��
(2)������У�Ϊ��ʹ��Ӧ��50��60���½��У��ɲ��õķ�����________________________________��
(3)�������ϴ�ӡ������������Ӧʹ�õ�������____________________��������дֲ�Ʒ��5%NaOH��Һϴ�ӵ�Ŀ����__________________________________________________��
(4)ʵ�����Ʊ����øĽ�װ�ý��б������ȡ����Ӧ����ʵ��װ��ͼ������AΪ�ɾ���֧�ܵ��Թ��Ƴɵķ�Ӧ�����������¶˿���һ��С�ף�����ʯ���ޣ��ټ���������м�ۡ�
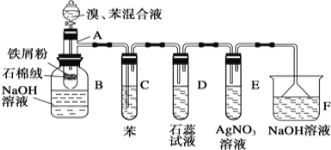
��Ӧ����A����μ�����ͱ��Ļ��Һ���������ھͷ�����Ӧ��д��A����������Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
(5)�Թ�C�б���������_________________________����Ӧ��ʼ�۲�D��E���Թܣ�����������Ϊ___________________________________��________________________________��
(6)��Ӧ2��3min����B�е�NaOH��Һ��ɹ۲쵽��������_______________________________��
(7)ͬѧ����Ϊ������������װ����D��E���Թ��еĵ���Ӧ����Һ���£�ʵ����������ԣ�������ͬѧ�ĸĽ����飺______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ü״�(CH3OH)�Ʊ�һЩ�߸���ֵ��Ʒ����Ŀǰ�о����ȵ㡣
��1���״���ˮ���������������Ƶ���������Ӧ��Ҫ�������£�
��Ӧ��. CH3OH(g)��H2O(g)![]() 3H2(g)��CO2(g) ��H1
3H2(g)��CO2(g) ��H1
��Ӧ��. H2(g)��CO2(g)![]() H2O(g)��CO(g) ��H2= a kJ��mol-1
H2O(g)��CO(g) ��H2= a kJ��mol-1
��Ӧ��. CH3OH(g)![]() 2H2(g)��CO(g) ��H3= b kJ��mol-1
2H2(g)��CO(g) ��H3= b kJ��mol-1
��Ӧ��. 2CH3OH(g)![]() 2H2O(g)��C2H4(g) ��H4= c kJ��mol-1
2H2O(g)��C2H4(g) ��H4= c kJ��mol-1
�١�H1=______kJ��mol-1 ��
�ڹ�ҵ�ϲ���CaO������ǿ����ķ�����������Ч��߷�Ӧ�������IJ��ʣ���ͼ1�����������CaO����������ʵ�ԭ��______��
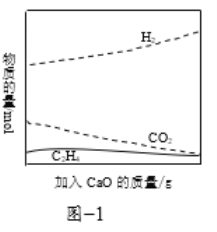
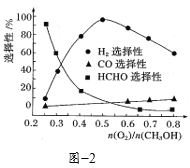
����һ���������������������״���������ԭ������![]() �Է�Ӧ��ѡ����Ӱ������ͼ2��ʾ(ѡ����Խ���ʾ���ɵĸ�����Խ��)���Ʊ�H2ʱ��ÿ���
�Է�Ӧ��ѡ����Ӱ������ͼ2��ʾ(ѡ����Խ���ʾ���ɵĸ�����Խ��)���Ʊ�H2ʱ��ÿ���![]() =______����
=______����![]() = 0.25ʱ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ______��
= 0.25ʱ��CH3OH��O2��������Ҫ��Ӧ����ʽΪ______��
��2����V2O5Ϊԭ�ϣ�����������-�״���ԭ�����Ʊ�VO2����������1000kW�£�ȡ��ͬ�����ķ�Ӧ����뷴Ӧ���У��ı䷴Ӧ�¶ȣ����ַ�Ӧʱ��Ϊ90min����Ӧ�¶ȶԸ�������������������Ӱ��������ͼ3��ʾ���¶ȸ���250��ʱ��VO2�����������½���ԭ����______��
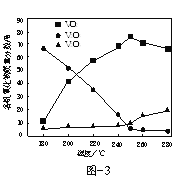
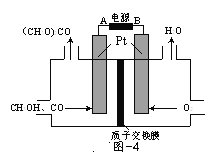
��3���Լ״�Ϊԭ�ϣ�����ͨ���绯ѧ�����ϳ�̼�������[(CH3O)2CO]������ԭ����ͼ4��ʾ��
�ٵ�Դ�ĸ���Ϊ______(�A����B��)��
�������ĵ缫��ӦʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ���������(��������δ���)��
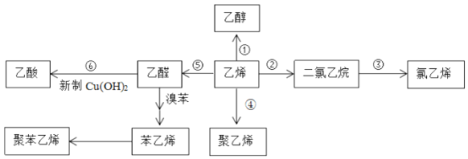
(1)�Ҵ��������ᷴӦ�����й���ζ�����ʣ�������Ϊ____________��
(2)д����Ӧ���ͣ���_____________����______________��
(3)��Ӧ�Ļ�ѧ����ʽ��____________________________________��ʵ��������______________________________��
(4)��Ӧ����KOH���Ҵ���Һ������������������ϩ�ķ���ʽΪ��____________________________________________________________________��
(5)������ϩ�����������ŵ�����_____________________________________��
(6)����ϩ�ϳɾ۱���ϩ�Ļ�ѧ����ʽ��__________________________________________��
(7)���Ҵ��ͱ���ϩΪԭ�Ϻϳ��л���![]() ��д���ϳ�·��ͼ_____________________________��(�ϳ�·�߳��õı�ʾ��ʽΪ��A
��д���ϳ�·��ͼ_____________________________��(�ϳ�·�߳��õı�ʾ��ʽΪ��A![]() B
B![]()
![]() Ŀ�����)
Ŀ�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Fe3O4�к���Fe����Fe������Fe3O4/PdΪ�����ϣ���ʵ����H2�������Է�ˮ�е��°���NO2-���䷴Ӧ������ͼ��ʾ������˵����ȷ���ǣ� ��
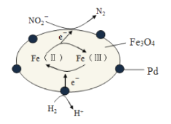
A.Pd������
B.Fe������Fe�����ת�����˴��ݵ��ӵ�����
C.��Ӧ������NO2-��Fe��������ΪN2
D.�ø÷�������ˮ���pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������Ӧ��(A)Cu2++Zn=Cu+Zn2+(B)2FeCl3��Cu=2FeCl2+CuCl2��
(1)�ֱ�����ʵ��װ��ͼ��ע���������Һ���ƺ����������ϣ����������������___��___��
(2)д�����е缫��Ӧʽ��
��(A)��___�Ǹ�����������Ӧʽ��___��
��(B)��___�Ǹ�����������Ӧʽ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼��þ������һ�����͵��������β����е���ǿ���ϡ�
��1���ϳɸ����ʵIJ������£�
����1������0.5mol��L-1 MgSO4��Һ��0.5mol��L-1 NH4HCO3��Һ��
����2������Ͳ��ȡ500mL NH4HCO3��Һ��1000mL������ƿ�У��������������¶ȿ�����50�档
����3����250mL MgSO4��Һ��μ���NH4HCO3��Һ�У�1min�ڵμ�����ð�ˮ������ҺpH��9.5��
����4������1h���ˣ�ϴ�ӡ�
����5����40�����ո������и���10h����̼��þ�����Ʒ��MgCO3��nH2O n=1��5����
�ٲ���2�����¶���50�棬�Ϻõļ��ȷ�����_________��
�ڲ���3����MgCO3��nH2O�����Ļ�ѧ����ʽΪ__________��
�۲���4��������Ƿ�ϴ�Ӹɾ��ķ�����__________��
��2���ⶨ���ɵ�MgCO3��nH2O�е�nֵ��
����1.000̼��þ���룬������ͼ��ʾ�Ĺ��ƿ�м�������ˮ��������ϡ�����뾧�뷴Ӧ�����ɵ�CO2��NaOH��Һ���գ��������·�Ӧ4��5h����Ӧ���ڽ��¶�����30�棬����ձ��е���Һ����֪Ũ�ȵ�����ζ������CO2���������ظ���������2�Ρ�
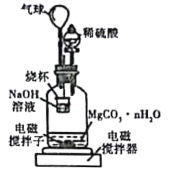
��ͼ�������������_________��
��������Ӧ����Ҫ���µ�30�棬��ҪĿ����______��
�۲��ÿ7.8000g̼��þ���������״����CO2Ϊ1.12L����nֵΪ_______��
��3��̼��þ���������þ���ã�Ϊ�ⶨij��þ����Ҫ�ɷ���̼��þ��������̼���������������裩�����ĺ�������ʵ���ҷֱ��ȡ12.5g��þ����Ʒ���ڹ�����ϡ���Ტ��ȫת�Ƶ���ƿ�У�����ָʾ������0.010mol/L H2O2��Һ���еζ���ƽ�вⶨ���顣����H2O2��Һ��������������ʾ��
ʵ���� | 1 | 2 | 3 | 4 |
����H2O2��Һ���/mL | 15.00 | 15.02 | 15.62 | 14.98 |
��H2O2��ҺӦװ��_________��������ʽ��������ʽ�����ζ����С�
�ڸ��ݱ������ݣ��ɼ������þ������Ԫ�ص���������Ϊ_________ %������С�������λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E����ǰ������ԭ�������������������Ԫ�ء�A��Dͬ�����������ֳ���������DA2��DA3����ҵ�ϵ������C2A3��ȡ����C��B��E��������ֻ��2�������⣬�������ȫ������Eλ��Ԫ�����ڱ���ds�����ش��������⣺
(1)B��C�е�һ�����ܽϴ����________����̬Dԭ�Ӽ۵��ӵĹ������ʽΪ________________��
(2)DA2���ӵ�VSEPRģ����____________��
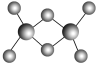
(3)ʵ����C����Ԫ���γɵĻ������ʵ�����ΪC2Cl6�������ģ����ͼ��ʾ����֪C2Cl6�ڼ���ʱ���������������NaOH��Һ��Ӧ������Na[C(OH)4]��
��C2Cl6����________(�������)���壬����Cԭ�ӵ��ӻ��������Ϊ________�ӻ���
��[C(OH)4]���д��ڵĻ�ѧ����____________________________________________��
(4)����A������ͬ�������壬���зе�ߵ���__________ (�����ʽ)��ԭ����______________________________________________________________
(5)D��E���γɻ����ᄃ��ľ�����ͼ��ʾ ��
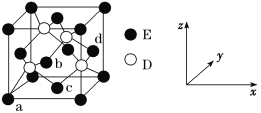
���ڸþ����У�E����λ��Ϊ________��
��ԭ����������ɱ�ʾ�����ڲ���ԭ�ӵ����λ�á���ͼ�����У�ԭ���������aΪ(0,0,0)��bΪ(1/2,0,1/2)��cΪ(1/2,1/2,0)����d���������Ϊ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com