
�������ڿ�����һ�㶼�ױ���������Ħ����[(NH4)2SO4��FeSO4��6H2O]��һ���������Ҫ�ȶ���������ʱ�ֽ��ױ���������ʵ���ҵ��Ʊ�ԭ��Ϊ:FeSO4+(NH4)2SO4+6H2O=(NH4)2SO4��FeSO4��6H2O��
��ͼΪ��ȡĦ���εļ�Ҫ���̣�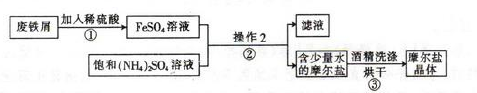
��ش���������:
��1��������з�Ӧ����Ӧ��������ҡ�λ���裬Ŀ���� ��
��2��������еIJ����Ǽ��������� �� ��Ϊʲô���ܼ�������? ��
��3��Ħ������NH4+��Fe2+��SO42-�ļ���:
�ټ�ͬѧ���������ʪ��� ��ֽ��ϡ����� ��Һ���������������ӡ�
����ͬѧ����������е�Fe2+��������KSCN��Һ�� ��Ԥ�ڵ�����ͽ����� ��
��ʵ�ʲ����У���ͬѧ����KSCN��Һʱ��������Һ���dz��ɫ�����������Լ��ķ�������˻��ɡ���ͬѧ������ϸ˼����Ϊ����ͬѧ�ķ����ǿ��еģ�������Ϊ ��
��4����ͬѧ���ⶨĦ������Fe2+�ĺ���������ȡ��4. 0gĦ������Ʒ������ˮ������������ϡ���ᣬ��0.20mol/L��KMnO4��Һ�ζ�������KMnO4��Һ10.00mL
�ٱ�ʵ���ָʾ���� (����ĸ)��
| A����̪ | B��ʯ�� | C������ | D������Ҫ |
��1����ֹFe2����������1�֣�
��2����ȴ�ᾧ�����ˣ�Ħ���������ֽ��ױ���������1�֣�
��3���ٺ�ɫʯ�������������1�֣�
����ˮ����˫��ˮ����1�֣�������KSCN��Һ�����������ټ�����ˮ����Һ��죬��֤����Һ�к���Fe2����2�֣�
�ۼ���������ˮ����Һ��ɫ���������֤����Һ�к���Fe2����1�֣�
��4����D��1�֣�
����ʽ��1�֣�
�۵������һ��KMnO4��Һҡ�Ⱥ���ƿ�е���Һ��dz��ɫ��Ϊdz��ɫ����30 s�ڲ���ɫ��2�֣�
��14%��2�֣�
���������������1����������ҡ�λ���裬��ֹFe2����������
��2������Ҫ��ȴ�ᾧ�����ˣ�Ħ���������ֽ��ױ�������
��3����������ٺ�ɫʯ���������������ˮ����˫��ˮ��������KSCN��Һ�����������ټ�����ˮ����Һ��죬��֤����Һ�к���Fe2�����ۼ���������ˮ����Һ��ɫ���������֤����Һ�к���Fe2����
��4����KMnO4��Һ������ɫ������Ҫָʾ����
�����ԡ��������Լ�Ӧ��ѡ����ʽ�ζ��ܡ�
�۵������һ��KMnO4��Һҡ�Ⱥ���ƿ�е���Һ��dz��ɫ��Ϊdz��ɫ����30 s�ڲ���ɫ��
�ܸ��ݵ����غ�KMnO4~5 Fe2+������n(Fe2��)=5��10��10-3 L��0.20mol/L=10-2 mol,Fe2+�İٷֺ���Ϊ10-2 mol��56g/mol��4.0g��100%=14%��
���㣺����Ԫ�ؼ��仯�������ʡ���ѧʵ��֪ʶ����ѧ��Ӧ�ļ��㡣


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijѧϰС����ͨ��ʵ��̽���̷���FeSO4��7H2O���ȷֽ�IJ��
��ʵ��ǰ����
��1�����۷��� С���Ա�������Ϸ�������Ϊ���зֽ���ﲻ���ܵ��� ��
a��Fe2O3��SO3��H2O b��Fe2O3��SO2��SO3��H2O
c��FeO��Fe2O3��SO2��SO3��H2O
��2���������� ��ѹ��SO3�۵�16��8�棬�е�44��6��
��ʵ��̽����
������Ͽ��ܵ���ϲ��룬��ѧϰС���������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ���
��3��ʵ�����
���������Ӻ��װ��A��B�����ԵIJ���Ϊ ��
��ȡһ�����̷���������A�У�ͨ��N2������װ���ڵĿ������ر�k���þƾ��Ƽ���
˫ͨ�ܡ�
�۹۲쵽A �й��������ɫ��B���Թ��ռ�����ɫҺ�壬C����Һ��ɫ��
�ܴ�A�з�Ӧ��ȫ����ȴ�����º�ȡ������Ӧ��������Թ��У����������ܽ⣬ȡ
�������뼸��KSCN��Һ����Һ���ɫ��
����Bװ�õ��Թ��е��뼸��BaCl2��Һ����Һ����ǡ�
( 4��ʵ��������
����1��B���ռ�����Һ���� ��
����2��C����Һ��ɫ������֪�������� ��
����3���ۺϷ�������ʵ��ۺܿ͢���֪�������һ����Fe2O3��
��ʵ�鷴˼��
��5����ָ����С����Ƶ�ʵ��װ�õ����Բ��㣺 ��
��6���ֽ��Ĺ����п��ܺ�������FeO��ȡ����ʵ����������ܽ�����Һ�������Թ��У�
ѡ��һ���Լ����𣬸��Լ�����ʵ��� ��
a����ˮ��KSCN��Һ b������KMnO4��Һ c�� H2O2 d�� NaOH��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
I������ռ������Ҫ�Ļ���ԭ�ϡ�
��1��������ͼ��ʾװ�ÿɼ��֤��������̼���ռ���Һ�����˷�Ӧ����A��B���ӣ���ֹˮ�У�����ͷ�ι��е�Һ�強����ƿ����ʱ��ʵ��������___________________��
�������������䣬��A��C���ӣ��ɹ۲쵽��������__________________________��
��2����NaOH��Һ��ͨ��һ����CO2���ᾧ��õ���ɫ���壬�ð�ɫ�������ɿ����ǣ�
A��NaOH��Na2CO3��B����������������C������������������D����������������
��3�����ʵ��ȷ����2���а�ɫ�����д���A���е������ӣ�
| ʵ����� | ʵ������ | ���� |
| ��ȡ������ɫ�������Թ��У�������ˮ�ܽ⣬�ټ�����BaCl2��Һ | | |
| �� | | |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧ��ȤС��Ϊ̽������Ũ����ķ�Ӧ,�����ͼ1��ͼ2��ʾװ�ý���ʵ�顣 
��1����˵����SO2���������ʵ�������� ��
��2��ͼ2�е�����e����Ҫ����Ϊ ��
��3������װ����ͼ2�е�NaOH��Һ������SO2β������ֹ��Ⱦ���罫�����Ϊ����KMnO4��Һ��ͬ�����ԴﵽĿ�ģ���д������KMnO4��Һ��SO2��Ӧ�Ļ�ѧ����ʽ��
��
��4���Ա�����ʵ��װ�ã����ѷ���ͼ2װ�ó����ܸ��õ������ж�����SO2��ֹ����Ⱦ�����⣬����һ���dz����Ե��ŵ㣬����Ϊ�� ��
��5����Ӧһ��ʱ���ֹͣ��Ӧ������ȴ���ý�ͷ�ι���ȡA�Թ��е���Һ���뵽����ˮ����Ϊ�����������������������ӵijɷ����������ֿ��ܣ�
��ֻ����Fe3+����ֻ����Fe2+�� ����Fe3+����Fe2+��
Ϊȷ����Һ�ijɷ֣�ѡ�������Լ���
| A��ϡHCl��Һ | B��ϡ���� | C��KSCN��Һ | D������KMnO4��Һ |
| ʵ�鲽�� | ʵ�������� |
| 1��ȡһ֧�ྻ���Թܣ��μ�1-2mL��������Һ�������Թ��еμӼ���KSCN��Һ | ��1�� ����˵��������� ��2�� ����˵����Һ�д���Fe3+������������ |
| 2�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪ij���������к���NaCl���ʣ�Ϊ�ⶨ�����д��������������������ͼ�е�װ�ý���ʵ�顣����ʾ����ʯ������ʯ�����������ƵĻ�����������ˮ�Ͷ�����̼��
��Ҫʵ�鲽�����£��� ��ͼ��װ������������װ�õ�������
�� ��10.0 g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ
�� ����ʢ�м�ʯ�ҵ�U�ܵ��������õ�20.0g
�� �ӷ�Һ©������6mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ
�� �ӵ���A����������һ�����Ŀ���
�� �ٴγ���ʢ�м�ʯ�ҵ�U�ܵ��������õ�22.0g
�� �ظ�����ݺ͢IJ�����ֱ��U�ܵ������������䣬Ϊ22.2g
����պͻش����⣺
��1��װ���и����B������ _________________________�����û�����Ӹø���ܣ����ԵĽ�� (��ƫ�ߡ�ƫ�ͻ䣩��
��2���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ�� (��ƫ�ߡ�
ƫ�ͻ䣩��
��3������ݵ�Ŀ���� ___________________�����û�н��в���ݵIJ��������ԵĽ��________________����ƫ�ߡ�ƫ�ͻ䣩��
��4�������д������������Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ������е�Ĺ�������ʾ��ͼ����
���������գ�
(1)����ˮ���������A��B������(A��Դ��ʯ��Ҥ��������B�Ļ�ѧʽ_________��
(2)�ڹ��˺���Һ��Ҫͨ��C��D���壬����ͨ���C������_________(�ѧʽ��,ԭ����_________��
(3)ͨ��C��D���������Ӧ�Ļ�ѧ����ʽ��_________��
(4)�ܹ��˺�����Һ��ͨ����,����ϸСʳ�ο�������������Ʒ________ (��д��ѧʽ����ͨ���������ϸСʳ�ο�����������_________��
(5)д���ݶ��շ�����Ӧ�Ļ�ѧ����ʽ_________����Ʒ�����к���̼�����ƣ����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�����������������̼�����Ƶ���ͯ������(NaHCO3)�� _________ (ע������ʽ�����õ��йط��ŵĺ��壩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ����С��Ϊ�ⶨij̼���ƺ�̼�����ƻ������̼���Ƶ������������ס�������ͬ
ѧ�ֱ�������������ʵ�顣
��������ͬѧ�ó�����������������ͼ��ʾ��ʵ�����̽���ʵ�飺[��֪Ba(HCO3)2������ˮ]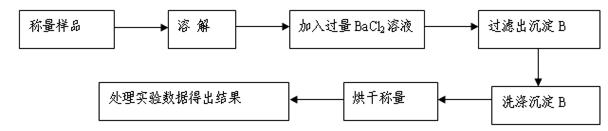
��1��ʵ��ʱ�����˲����У����˲�������©���⣬��Ҫ�õ��IJ�������Ϊ ��
��2��ϴ�ӳ���B�IJ����� ��
��3����ʵ���в����Ʒ����Ϊm g����������Ϊn g����̼���Ƶ���������Ϊ____________��
����������ͬѧ����Ҫʵ������ͼ���£�
������ͼ��ʾװ�ý���ʵ�飺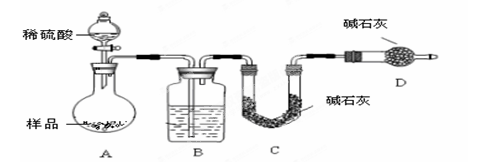
��4����ʵ����װ��Bʢ�ŵ�������_____________________����Һ©���� ����ܡ����ܡ������������ϡ�������ʵ�顣
��5����C��װ��ʯ�������վ���������塣
����Ʒ��̼���Ƶ���������Խ����ʵ�������վ���������ĸ�����ڳ����������ǰ���������
____________________�����Խ����ԽС�����仯����
��Dװ�õ�������_________________________��
��6���е�ͬѧ��ΪΪ�˼���ʵ�����ڷ�Ӧǰ��Ҫͨ��N2������ͼ������Ӧ��ͨ��N2��Ŀ����____
__________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ʯ����Ҫ�ɷ���K2SO4��Al2(SO4)3��2Al2O3��6H2O������������Fe2O3���ʡ���������ʯ�Ʊ������������������£�
(1)����¯�з�����Ӧ�Ļ�ѧ����ʽΪ2Al2(SO4)3+3S 2Al2O3+9SO2���÷�Ӧ����������_______��������l molAl2O3����ת�Ƶĵ�����Ϊ__________________��
2Al2O3+9SO2���÷�Ӧ����������_______��������l molAl2O3����ת�Ƶĵ�����Ϊ__________________��
(2)�����ܽ�ʱ����Ӧ�����ӷ���ʽΪ___________________________________________��
(3)ĸҺ��������Ҫ�ɷֵĻ�ѧʽΪ_____________________________________________��
(4)����״����1.12L¯��ͨ��100mL 0��5mol��L-1NaOH��Һ�У��õ�һ��������Һ�������Һ�и�������Ũ���ɴ�С������˳��Ϊ____________________________��
(5)�����������Ҫ�ɷֵķ�����_____________________________________________________(д���������衢������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������������������Ӧʱ���������Ԫ�صĻ��ϼ۲��ᷢ���仯���ǣ�������
| A��ϡ���� | B��ϡ���� | C��Ũ���� | D��Ũ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com