
����Ŀ������N�����ף�P�����飨As����VA��Ԫ�صĻ��������о�������������Ҫ��;����ش��������⣺
��1����̬��ԭ�ӵĵ����Ų�ͼΪ________________������ԭ��ͬ�����Һ��е�δ�ɶԵ�������ͬ��Ԫ����________�֡�
��2��(SCN)2�����и�Ԫ�صĵ縺���ɴ�С��˳��Ϊ______________����Ԫ�ط��ű�����������������������������Ϊ____________��(SCN)2����Cu2+�γ�����������_______________________________��
��3��CO2��N2O�ĵȵ����壬N2O������ԭ�ӵ��ӻ��������Ϊ_______________��
��4�����һ���������׳�����˪�����ҹ�������Ա�о�������˪��Ѫ�������Ե��������ã���ṹ��ͼ1��ʾ������˪���Ļ�ѧʽΪ___________������˪����һ����������ת����Na3AsO4��Na3AsO4�������ӵĿռ乹��Ϊ____________________��
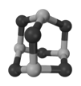
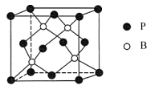
ͼ1 ͼ2
��5��������һ����ĥ���ϣ��侧���ṹ��ͼ2��ʾ��������һ��Bԭ����Χ���������Pԭ����____������B��Pԭ�Ӱ뾶�ֱ�Ϊr1pm��r2pm�������ӵ�����ֵΪNA�������ܶ�Ϊd g/cm3����������ԭ�ӵ����ռ��������İٷ���Ϊ________ ��100% ���ú�d��r1��r2 �Ĵ���ʽ��ʾ����
���𰸡�![]() 2 N>S>C 5: 4 SCN-���й¶Ե���,Cu2+�пչ��,�������γ������� sp�ӻ� As4O6 ���������� 4
2 N>S>C 5: 4 SCN-���й¶Ե���,Cu2+�пչ��,�������γ������� sp�ӻ� As4O6 ���������� 4 ![]() dNA(r13+r23)
dNA(r13+r23)![]() 10-30]/63
10-30]/63![]()
��������
��1����Ϊ15��Ԫ�أ���̬��ԭ�ӵĵ����Ų�ͼΪ![]() ����3��δ�ɶԵ��ӣ���ԭ��Ҳ��3��δ�ɶԵ��ӣ�����ͬ�����Һ��е�δ�ɶԵ�������ͬ��Ԫ���з��������֡��𰸣�
����3��δ�ɶԵ��ӣ���ԭ��Ҳ��3��δ�ɶԵ��ӣ�����ͬ�����Һ��е�δ�ɶԵ�������ͬ��Ԫ���з��������֡��𰸣�![]() ��2��
��2��
��2��Ԫ�����ڱ��У�ͬ���ڴ�����Ԫ�صĵ縺������ǿ��ͬ������ϵ���Ԫ�صĵ縺��������N��CΪͬ����Ԫ��,�縺��N>C,��ΪH2CO3Ϊ���ᣬH2SO4Ϊǿ�ᣬ���Էǽ�����S>C,�縺��S>C��N�ĺ�������Ų�Ϊ�����״̬�����Ե縺��N>S������(SCN)2�����и�Ԫ�صĵ縺���ɴ�С��˳��Ϊ��N>S>C��(SCN)2�ĽṹʽΪN![]() C-S-S-C
C-S-S-C![]() N, �������������ĸ�����Ϊ5: 4��SCN-���й¶Ե���,Cu2+�пչ��,�������γ������ӡ��𰸣�N>S>C��5: 4��SCN-���й¶Ե���,Cu2+�пչ��,�������γ������ӡ�
N, �������������ĸ�����Ϊ5: 4��SCN-���й¶Ե���,Cu2+�пչ��,�������γ������ӡ��𰸣�N>S>C��5: 4��SCN-���й¶Ե���,Cu2+�пչ��,�������γ������ӡ�
��3��CO2��N2O�ĵȵ����壬���ݵȵ���ԭ����֪��N2O������ԭ���������̼������ԭ��һ�����۲���Ӷ���Ϊ2�Ҳ����µ��Ӷԣ�Ϊsp�ӻ����𰸣�sp�ӻ���
��4������ͼ1�ṹ��֪,��˪�����ں���6����ԭ��,4����ԭ��,�仯ѧʽΪAs4O6��Na3AsO4��������ΪAsO43-��AsO43-�к��еŵ��Ӷ�����0,����ԭ����4�������I���������ͬ����Ϊ���������Ρ��𰸣�As4O6�����������Ρ�
��5������ͼ֪��Bԭ������4��Pԭ�ӡ��þ�����Bԭ�Ӹ���Ϊ4��Pԭ�Ӹ���Ϊ8![]() 1/8+6
1/8+6![]() 1/2=4,����������ԭ�����Ϊ4/3
1/2=4,����������ԭ�����Ϊ4/3![]() (r13+r23)
(r13+r23)![]() 4
4![]() 10-30cm3,�ܶ�d=(42/NA
10-30cm3,�ܶ�d=(42/NA![]() )/V,�����V=168/dNA cm3,������ԭ�ӵ�����ռ��������İٷ���=����ԭ�ӵ����/���������=[4/3
)/V,�����V=168/dNA cm3,������ԭ�ӵ�����ռ��������İٷ���=����ԭ�ӵ����/���������=[4/3![]() (r13+r23)
(r13+r23)![]() 4
4![]() 10-30cm3]/ 168/dNA cm3/span>
10-30cm3]/ 168/dNA cm3/span>![]() =
=![]() dNA(r13+r23)
dNA(r13+r23)![]() 2
2![]() 10-30]/63
10-30]/63![]() ;�𰸣�4��
;�𰸣�4��![]() dNA(r13+r23)
dNA(r13+r23)![]() 10-30]/63
10-30]/63![]() ;
;



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨ̼���ƴ��ȣ�ֻ���������Ȼ��ƣ���ѧ���������������ʵ�鷽����
[����1]��ȡ![]() ��Ʒ������С�ձ��У�������ˮ��ȫ�ܽ⣻��С�ձ��м�����������������Һ�����ˣ�ϴ�ӡ������������������������Ϊ19.700�ˣ����㡣
��Ʒ������С�ձ��У�������ˮ��ȫ�ܽ⣻��С�ձ��м�����������������Һ�����ˣ�ϴ�ӡ������������������������Ϊ19.700�ˣ����㡣
��1��д�����ɳ����ķ�Ӧ����ʽ__________________��
��2�����˲�����Ҫ�IJ�������__________________��
��3������̼���Ƶ���������Ϊ______________��������λС������
[����2]����ͼװ�ã���Һ���ⶨ![]() ���������������Ʒ������
���������������Ʒ������![]() ���������
���������
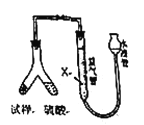
��4��Ϊ�˼�С�ⶨ![]() ��������������м����Һ��
��������������м����Һ��![]() Ϊ______���ѧʽ����
Ϊ______���ѧʽ����
��5��ͨ��ʵ�飬��ø�������̼���Ƶ���������ƫ�ߣ��������������ԭ�������______��
A.�ⶨ�������ʱδ��ȴ������
B.�������������ǰδ��Ũ�������
C.![]() �������з�Ӧ���ɵ�����
�������з�Ӧ���ɵ�����
D.��Ӧ���������ʱˮ�ܵ�ˮ����������ܵ�ˮ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ȼ���еĺ����ḻ������������������Ӧ�ù㷺��
��1���������ڱ��е�λ��Ϊ______��
��2�������Լ�[Fe(NO)CN)5]2�������ڼ���S2�������߷�Ӧ����ɫ��Һ�������������廥Ϊ�ȵ����壬��֪�����Լ�[Fe(NO)(CN)5]2����FeΪ+2�ۣ����̬���ӵ���Χ�����Ų�ʽΪ_______��
��3��K4[Fe(CN)6]��Һ�����ڼ���Fe3+��K��C��N�ĵ�һ�������ɴ�С��˳��Ϊ______��K4[Fe(CN)6]�ڽ��������������ĸ�����Ϊ______��
��4����ï���������������ϩ��������Fe2+������ɵ��������ͻ������![]() �������ϩ����(
�������ϩ����(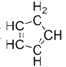 )��̼ԭ�ӵ��ӻ���ʽΪ_______��
)��̼ԭ�ӵ��ӻ���ʽΪ_______��
��5����ҵ������ڵ�FeO��Fe2O3ұ���ߴ�����FeO��Fe2O3��ȣ�_____�۵�ߣ�����Ҫԭ����_______��
��6�������ʾ����ڲ�ͬ�¶�����������Ҫ�Ķѻ���ʽ������������(A)����������(B)���ٶ�Feԭ�Ӱ뾶���䣬�����ֶѻ��У���λ��֮��NA�UNBΪ_______�����ܶ�֮����A�U��BΪ_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ��֣�H2S�Ǽ�NO��CO֮�������������ϵ�����źŷ��ӣ������в���������źŴ��ݡ�����Ѫ�ܼ����Ѫѹ�Ĺ��ܡ��ش��������⣺
��1����ͼ��ͨ���Ȼ�ѧѭ���ڽϵ��¶�����ˮ������ֽ��Ʊ������ķ�Ӧϵͳԭ����

ͨ�����㣬��֪ϵͳ����ϵͳ����������Ȼ�ѧ����ʽ�ֱ�Ϊ________________��______________���Ƶõ���H2�����������ٵ���_____________���ϵͳ������ϵͳ��������
��2���ʻ���(COS)����Ϊһ����ʳѬ�������ܷ�ֹijЩ���桢�߳�������Σ����H2S��CO2�ڸ����·�����Ӧ��H2S(g)+CO2(g)![]() COS(g) +H2O(g)����610 Kʱ����1 mol CO2��1 mol H2S����2 L�Ŀո�ƿ�У���Ӧƽ���ˮ�����ʵ�������Ϊ0.02��
COS(g) +H2O(g)����610 Kʱ����1 mol CO2��1 mol H2S����2 L�Ŀո�ƿ�У���Ӧƽ���ˮ�����ʵ�������Ϊ0.02��
��H2S��ƽ��ת����![]() =_______%����Ӧƽ�ⳣ��K=________��
=_______%����Ӧƽ�ⳣ��K=________��
����620 K�ظ����飬ƽ���ˮ�����ʵ�������Ϊ0.03��H2S��ת����![]() _____
_____![]() ���÷�Ӧ��
���÷�Ӧ��![]() H_____0�����>������<����=����
H_____0�����>������<����=����
����Ӧ�����ٷֱ�����������壬��ʹH2Sת�����������________�����ţ�
A��N2 B��H2S C��COS D��CO2
��3��25�棬��0.10 mol��L-1 H2S��Һ�У�ͨ��HCl��������NaOH�����Ե�����ҺpH����ҺpH��c(S2-) ��ϵ����ͼ��������Һ����ı仯��H2S�Ļӷ�����

������ĵ��뷽��ʽΪ__________________________��
����������Һ�м���CuSO4��Һʱ������ĵ���ƽ����______�������������������ƶ���c(S2��)_____________����������������������������С������
�۵�������ҺpH=13ʱ����Һ�е�c( H2S ) + c( HS��)=_____mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺

��ѡ�Լ���Na2SO4��Һ��K2SO4��Һ��K2CO3��Һ������
��1�������ٵ�������___�������ڵ�������___��
��2���Լ�a��___������B��___�����ѧʽ��
��3�����ɳ���A�����ӷ�Ӧ����ʽΪ��__�����Լ�b�������Ļ�ѧ��Ӧ����ʽΪ��___��
��4���÷����ܷ�ﵽʵ��Ŀ�ģ�___�������ܣ�Ӧ��θĽ������ܣ����ʲ��ûش�___��
��5����Ҫ�ⶨԭ�������KCl��BaCl2����������������Ҫȷ���������������⣬���ٻ�Ҫ��õ�������____��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���У�����ȷ���ǣ��� ��
�ٽ����ơ�����������������ȼ�գ����ɰ�ɫ����������ͭ˿��������ȼ�գ���������ɫ���Ȼ�ͭ����Һ�Ⱦ���������ˮ��Һ������ʹ�������ɫ������ɫ���ܾ��õ���ˮ��������������ȫ�ӷ�����ʣ�µľ���ˮ
A.��B.�٢�C.�٢ۢ�D.�٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ı�0.1molL��1��Ԫ����H2A��Һ��pH����Һ�е�H2A��HA����A2�������ʵ���������(x)��pH�ı仯��ͼ��ʾ[��֪����x��=c(x)/(c(H2A)+c(HA��)+c(A2��)])������������ȷ����

A. Ka2��H2A����������Ϊ10-4
B. NaHA��Һ�У�HA����ˮ������С��HA���ĵ�������
C. �ں�H2A��HA����A2������Һ�У���������NaOH���壬��(HA��)һ������
D. �������ʵ�����NaHA��Na2A���������ˮ�����õ���Һ����(HA��)����(A2��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�������ʵ�鷽�����Է���KCl��BaCl2���ֹ�������Իش��������⣺

��ѡ�Լ���Na2SO4��Һ��K2SO4��Һ��K2CO3��Һ������
��1�������ٵ�������___�������ڵ�������___��
��2���Լ�a��___������B��___�����ѧʽ��
��3�����ɳ���A�����ӷ�Ӧ����ʽΪ��__�����Լ�b�������Ļ�ѧ��Ӧ����ʽΪ��___��
��4���÷����ܷ�ﵽʵ��Ŀ�ģ�___�������ܣ�Ӧ��θĽ������ܣ����ʲ��ûش�___��
��5����Ҫ�ⶨԭ�������KCl��BaCl2����������������Ҫȷ���������������⣬���ٻ�Ҫ��õ�������____��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӻ�ѧ������չ����ѧ�ĸ�����֧������չ��������ѧ������ѧ��������Mo��2��C60���ӡ�2��p�����ᶡ����़�2��CO����������λ������װ�ij����ӽṹ��ͼ��ʾ��

(1)Mo���ڵ������ڵ�VIB�壬��������Ų���Cr���ƣ����Ļ�̬�۵����Ų�ʽ��___________������δ�ɶԵ�������___________����
(2)�ó������д��ڵĻ�ѧ��������___________��
A ���� B �м� C ���Ӽ� D ���
(3)�ó�����������CO�ṩ�µ��ӶԵ�ԭ����___________(��Ԫ�ط���)��p�����ᶡ�����������Cԭ�ӵ��ӻ���ʽ��___________��
(4)�ӵ縺�ԽǶȽ���CF3COOH������ǿ��CH3COOH��ԭ��___________��
(5)C60����ʯ��Ϊͬ�������壬�ӽṹ������֮��Ĺ�ϵ����C60���۵�Զ���ڽ��ʯ��ԭ����___________��
(6)��֪��ij�����и�ԭ�ӵ����λ�ÿ�����ͼ��ʾ��ԭ�������ʾ���������ж���ԭ�������Ϊ(0��0��0)��

��(Mo)��һ��������ϵ�ľ���ṹ�У�ÿ��������2��Moԭ�ӣ�����Moԭ��������(0��0��0)��(1/2��1/2��1/2)������������Ϣ���ƶϸþ����ԭ�Ӷѻ���ʽ��___________����֪�þ�����ܶ��Ǧ�g��cm��3��Mo��Ħ��������M g��mol��1�������ӵ�������NA�������о��������Moԭ�Ӻ�֮��ľ���Ϊ___________pm��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com