
����Ŀ�����������(NH2COONH4)��һ���ֽ⡢��ˮ��İ�ɫ���壬������CCl4��ʵ���ҿɽ����������̼���ﰱ��ͨ��CCl4�н����Ʊ�����ѧ����ʽΪ��2NH3(g)+CO2(g)=NH2COONH4(s) ��H��0��
�ش��������⣺
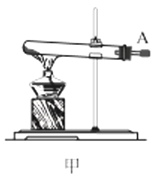
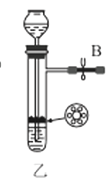

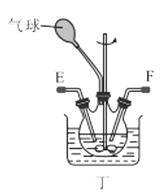
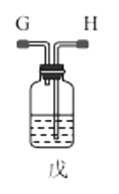
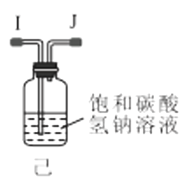
��1������װ�ü��Ʊ������Ļ�ѧ����ʽΪ__��
��2���������װ���������ԵIJ���__��
��3��ѡ��ͼ�е�װ���Ʊ���������泥������ӿڵ�����˳��Ϊ��B��__��__��EF��__��A��
��4����ӦʱΪ�����Ӱ�������淋IJ���������ƿ�ļ��ȷ�ʽΪ__��������ˮԡ��������ˮԡ���������������������__��
��5����װ�ö��Ļ�����з������Ʒ�ķ�����__����д�������ƣ���
��6��ȡ����������Ϊ̼����淋İ����������Ʒ11.730g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ��������������Ϊ15.000g������Ʒ�а�������淋���������Ϊ__����֪��Mr(NH2COONH4)=78��Mr(NH4HCO3)=79��Mr(CaCO3)=100������������3λ��Ч���֣���
���𰸡�Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O �н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ�棬���ã���Һ���ֲ��䣬��������������� I J H G D(C)��C(D) ��ˮԡ ƽ����ѹ���ռ���������� ���� 79.8%
CaCl2+2NH3��+2H2O �н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ�棬���ã���Һ���ֲ��䣬��������������� I J H G D(C)��C(D) ��ˮԡ ƽ����ѹ���ռ���������� ���� 79.8%
��������
(1)ʵ�������Ȼ�狀��������ƾ��干����ȡ������
(2)�н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ��仯�����
(3)ѡ��ͼ�е�װ���Ʊ���������泥���ȡ�Ķ�����̼�к���HCl��ˮ��������ȡ����ʱ�����к���ˮ������Ҳ��Ҫ��ȥ�������壻
(4)���������(NH2COONH4)��һ���ֽ⡢��ˮ��İ�ɫ���壬������ʵ����ʷ������������������ƽ������ѹǿ��
(5)������֪��Ϣ�����������(NH2COONH4)������CCl4������
(6)ȡ����������Ϊ̼����淋İ����������Ʒ11.730g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ��������������Ϊ15.000g��ͨ��̼ԭ���غ㣬������Ʒ�а�������淋�����������
(1)ʵ�������Ȼ�狀��������ƾ��干����ȡ����������ʽΪ��Ca(OH)2+2NH4Cl![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
(2)���װ���������ԵIJ������н�ֹˮ��B����©����ˮ��©���е�Һ������Թ��е�Һ�棬���ã���Һ���ֲ��䣬��������������ã�
(3)ѡ��ͼ�е�װ���Ʊ���������泥���ȡ�Ķ�����̼�к���HCl��ˮ������Ӧ��ͨ��װ�����еı���̼��������Һ��ȥHCl��Ȼ��ͨ�����е�Ũ�����ȥˮ��������ȡ����ʱ�����к���ˮ������ͨ��װ�ñ��еļ�ʯ�ң������ӿڵ�����˳��Ϊ��B��I J��HG��EF������DC��CD��A��
(4)���������(NH2COONH4)��һ���ֽ⡢��ˮ��İ�ɫ���壬���ɰ�������淋ķ�Ӧ�Ƿ��ȵģ�������Ҫ�����¶ȣ�����ˮԡ���������������ƽ������ѹǿ���������ն���δ��Ӧ�����壻
(5)���������(NH2COONH4)����ˮ��İ�ɫ���壬������CCl4�������ʹ�ù��˵ķ������룻
(6)ȡ����������Ϊ̼����淋İ����������Ʒ11.730g��������ʯ��ˮ��ִ�����ʹ̼Ԫ����ȫת��Ϊ̼��ƣ����ˡ�ϴ�ӡ��������������Ϊ15.000g��̼��Ƶ����ʵ���=![]() =0.1500mol������̼ԭ���غ㣬�����������Ʒ��̼�����ʵ���ҲΪ0.1500mol��̼����狀Ͱ�������淋����ʵ����ֱ�Ϊx��y����x+y=0.15��79x+78y=11.73����ã�x=0.03mol��y=0.12mol������Ʒ�а�������淋���������Ϊ
=0.1500mol������̼ԭ���غ㣬�����������Ʒ��̼�����ʵ���ҲΪ0.1500mol��̼����狀Ͱ�������淋����ʵ����ֱ�Ϊx��y����x+y=0.15��79x+78y=11.73����ã�x=0.03mol��y=0.12mol������Ʒ�а�������淋���������Ϊ![]() ��100%=79.8%��
��100%=79.8%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. NH3�Ǽ��Է��ӣ�������Nԭ�Ӵ���3��Hԭ������ɵ������ε�����
B. CCl4�ǷǼ��Է��ӣ�������Cԭ�Ӵ���4��Clԭ������ɵ������ε�����
C. H2O�Ǽ��Է��ӣ�������Oԭ�Ӳ���2��Hԭ������ֱ�ߵ��е㴦
D. CO2�ǷǼ��Է��ӣ�������Cԭ�Ӳ���2��Oԭ������ֱ�ߵ��е㴦
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
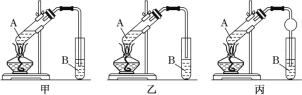
����Ŀ��ij��ѧ����С����ʵ������ȡ��������ʱ�����������£�
CH3COOH+C2H5OH![]() CH3COOC2H5+H2O�����ݲ�õ������������ͼ��ʾ��װ��(�г�װ�ú���)��ȡ�����������������������£�
CH3COOC2H5+H2O�����ݲ�õ������������ͼ��ʾ��װ��(�г�װ�ú���)��ȡ�����������������������£�
������ͼ��������ƿ�м���3mL�Ҵ�����ҡ������������3mLŨ���ᣬ�ڷ�Һ©����װ�������Ϊ3��2���Ҵ���������Һ��

����ԡ����������ƿ��һ���¶ȣ�Ȼ��ѷ�Һ©���еĻ��Һ�����ص���������ƿ�ﲢ���ַ�Ӧ�������һ���¶ȡ�
�۷�Ӧһ��ʱ�������ƿ�л������뱥��Na2CO3��Һ��������ҡ�����ֲ����з�Һ��
���ñ���ʳ��ˮ���Ȼ�����Һϴ�����㣬�ٷ�Һ�������������������ô�����������
�ݽ�����������ת����ͼ������A�У���ˮԡ�м��ȣ��ռ�74-80������ּ��ô�����ˮ
����ζ��ɫ��Һ�塣

������ĿҪ��ش�
(1)��ʵ����Ũ���������___��
(2)ͼ2������A��������___������������ˮ��___(��a��b)�ڽ��롣
(3)����ۺ͢��ж��õ��˷�Һ�������ڷ�Һ����ʱ����������Һ���Ƴ��IJ���������___��
(4)������и���������������ѡ�õĸ����Ϊ___(����ĸ)��
a.���������� b.��ˮNa2SO4c.��ʯ��
(5)��֪����(CH3CH2CH2CH2OH)���Ҵ��������ƵĻ�ѧ���ʣ���д�������Ĵ������Ļ�ѧ����ʽ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ����������һ�ݻ���Ϊ2L�������ڷ�����Ӧ��2A(g)+B(g2C(g)����֪��ʼͶ��4molA(g)��2molB(g)����2s����C��Ũ��Ϊ0.6mol/L������2s������Ũ�Ȳ��ٸı䡣����˵����ȷ����
A.2 s ��������A ��ʾ��ƽ����Ӧ����Ϊ 0.6 mol/(Ls)
B.2 s ��������B ��ʾ��ƽ����Ӧ����Ϊ 0.15mol/(Ls)
C.2 s ��ÿ�� 0.6 mol ������B ���ɣ�ͬʱ�� 0.6 mol ����C ����
D.2 s ʱ����B ��Ũ��Ϊ 0.7 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ԭ��Ϊԭ��������ϩ���ļ����������£�
![]()
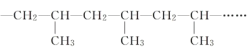
(1)�����ʹ�õķ����Ƿ�����������ԭ���и���ֵ�______��ͬ���з���Ĺ��̡�
(2)���������C4��C10 ���������������������ʳ��� 7 �֡�����C4H10 Ϊ��˵����ԭ��______��
(3)������У���ϩ���ĺϳɷ�Ӧ�����ϩ����ͬ���÷�Ӧ������_____�������ľ۱�ϩ������ͼ��ʾ�����ظ��ṹ��Ԫ��_________��
(4)��ϩ��һ����Ҫ�Ļ�������ԭ�ϣ����Ʊ�������������ת����ϵ��ͼ��
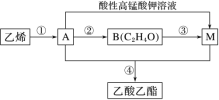
��֪��H2C==CHOH ���ȶ�
I.�ٵķ�Ӧ������______��
II.B�Ľṹ��ʽ��_______��
III.�ܵĻ�ѧ����ʽ��_______��
IV.ʵ������ȡ����������װ����ͼ��ʾ������ɱ�ʵ���װ����_____(����������������������)���Թ�B ��ʢ�ŵ�Һ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����̬Feԭ�ӵļ����Ų�ʽΪ______________________��
��2�������£�Fe(CO)5Ϊ��ɫҺ�壬�����ڷǼ����ܼ���д��CO�ĵ���ʽ______________��Fe(CO)5����������������֮��Ϊ______________��
��3��Ni������±��(SCN)2��Ӧ����Ni(SCN)2��Ni(SCN)2�е�һ����������Ԫ����_____________��(SCN)2��������ԭ�ӵ��ӻ���ʽ��_____________��
��4������ͭ���ڰ�ˮ�γ�[Cu(NH3)4](NO3)2������ɫ��Һ��
��[Cu(NH3)4](NO3)2�������ӵ����幹����_________________��
����NH3��Ϊ�ȵ������һ��������Ϊ_____________���ѧʽ����������һ����ѹǿ�£���õ��ܶȱȸ�ѹǿ�������ܶ��Դ������ԭ��__________��
��5����������ɿ��ɽ���ԭ������ά�ռ��жѻ����ɣ�����������ԭ�Ӳ���ͭ��ģʽ�ѻ���ԭ�ӿռ�������Ϊ74%������ԭ�ӵ���λ��Ϊ________________��
��6���������γɵ�ij�־����ṹ����ͼ��ʾ����������a��xpm��������ʵĻ�ѧʽΪ___________________��Aԭ�Ӿ���Bԭ������������������̾���Ϊ________________pm����x��ʾ���� �þ������ܶ�Ϊ____________g��cm-3���������ӵ�������NA��ʾ��
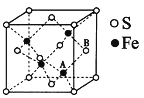
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶�ʱ����2L������X��Y��Z�����������ʵ����ʵ�����n������ʱ�䣨t���仯��������ͼ��ʾ����ͼ������
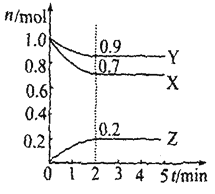
��1���÷�Ӧ�Ļ�ѧ����ʽΪ_________________��
��2����Ӧ��ʼ��2min����Z��ʾ��ƽ����Ӧ����Ϊ_____________��
��3������������˵��������Ӧ�ﵽƽ��״̬����_____________������ţ���
A��X��Y��Z�����ʵ���֮��Ϊ3��1��2
B����������ѹǿ����ʱ��ı仯���仯
C����λʱ����ÿ����3molX��ͬʱ����2molZ
D��������������������ʱ��ı仯���仯
E���������������ʵ�������ʱ��ı仯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ʽ��з���(�����)
��O2��O3��![]() C ��
C ��![]() C�۰�������עܱ���ˮ��H2��D2��T2���Ҵ�������Ѣ�
C�۰�������עܱ���ˮ��H2��D2��T2���Ҵ�������Ѣ�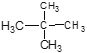 ��
��![]()
(1)��Ϊͬλ�ص���_________��
(2)��Ϊͬ�����������_________��
(3)��Ϊͬ���칹�����_________��
(4)����ͬһ���������_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����λ�����Ǧ(3PbO��PbSO4��H2O)���������������ɫ����ɫ��ĩ�����ȶ�������������Ҫ����������ϩ�����ȶ����������������ɿ�����Ǧ���м���������������Ǧ���ټ��������ƶ��Ƶá���100.0��Ǧ�ࣨ��Ҫ�ɷ�ΪPbO��Pb��PbSO4�ȣ�Ϊԭ���Ʊ����εĹ�����������ͼ��ʾ

��֪����1��Ksp(PbSO4)=1.82��10-8��Ksp(PbCO3)=1.46��10-13��
��2��Ǧ�������ᡢ�����Ἰ���������á�
��ش��������⣺
��1��д�������١�ת�����Ļ�ѧ����ʽ_________________________________
��2�������������1����������������Ҫ�ɷ�Ϊ_____________
��3��������������������ʺ�ѡ�õ���Ϊ___________��Ϊ����������ʣ��ɲ�ȡ�Ĵ�ʩ��____________________________________������д��һ����
��4�������������Ǧ�������Һ��c(Pb2+)=1.82��10-5mol��L-1�����ʱc(SO42-)=_________ mol��L-1
��5����ԭ�������ʵĽǶȷ��������̵��ŵ�Ϊ____________________________________��
��6����������ϳ������εĻ�ѧ����ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com