
����Ŀ�������е⺬���Ƚϸߣ��Ӻ�����ȡ��IJ�������:
(1)���ɺ����������յ�������________���������к��н϶�KI��������������ˮ��Ȼ����˵õ�������Һ��
(2)��������Һ�м��������H2O2�����Һ���õ��غ�ɫ���е��ʵ��ˮ��Һ����д�����ӷ�Ӧ����ʽ:________________________________��
(3)���������ˮ��Һ�м�������CCl4�������ã���I2��ת�뵽CCl4���У�������̽�_____________________������Ϊ____________________________��
(4)3I2��6KOH=5KI��KIO3��3H2O��1.5mol I2��ȫ��Ӧת�Ƶ��ӵ����ʵ���Ϊ_____mol���������뻹ԭ�������ʵ���֮��Ϊ___________��
(5)ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ6������:O2��K2Cr2O7��Cr2��SO4��3��H2SO4��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹���:H2O2��O2
�ٸ÷�Ӧ�У�������Ϊ____________����������Ϊ____________��
��д���÷�Ӧ�Ļ�ѧ����ʽ__________________________��
���õ����ŷ���ʾ������Ӧ�е���ת�Ʒ������Ŀ________________��
���𰸡����� H2O2+2I-+2H+=2H2O+I2 ��ȡ ����������Һ�ֿ����ϲ�Ϊ��ɫ���²�Ϊ�Ϻ�ɫ 2.5mol 5:1 K2Cr2O7 O2 K2Cr2O7+3H2O2+4H2SO4=Cr2(SO4)3+7H2O+3O2��+K2SO4 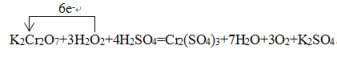
��������
��1��������������������
��2������������ԭ��Ӧ������д��
��3����ȡ���������Ȼ�̼���ܶȱ�ˮ���л���λ���²㣻
��4������������ԭ��Ӧ�����غ���м��㣻
��5������������ԭ��Ӧԭ������жϣ�������ָ��õ����ӵķ�Ӧ��һ����
(1)���ɺ����������յ��������������������к��н϶�KI��������������ˮ��Ȼ����˵õ�������Һ��
(2)��������Һ�к��е⻯�أ����������H2O2�����Һ�������������ǿ�����ԣ��õ��غ�ɫ���е��ʵ��ˮ��Һ�����ӷ�Ӧ����ʽH2O2+2I-+2H+=2H2O+I2��
(3)���������ˮ��Һ�м�������CCl4�������ã���I2��ת�뵽CCl4���У�������̽���ȡ������Ϊ����������Һ�ֿ����ϲ�Ϊ��ɫ���²�Ϊ�Ϻ�ɫ��
(4)3I2��6KOH=5KI��KIO3��3H2O���ⵥ�ʷ����绯��Ӧ��3mol�ⵥ������5mol��ԭ����⻯�أ�1mol�����������أ�ת�Ƶ�����Ϊ5mol����1.5mol I2��ȫ��Ӧת�Ƶ��ӵ����ʵ���Ϊ2.5mol������ϵ��֮�ȵ������ʵ���֮�ȣ�������������KIO3Ϊ1mol����ԭ��I2Ϊ0.5mol����ԭ����KIΪ5mol����������I2Ϊ2.5mol�����Ը÷�Ӧ�������뻹ԭ�������ʵ���֮��Ϊ5��1��
(5)ijһ��Ӧ��ϵ�з�Ӧ��������ﹲ6������:O2��K2Cr2O7��Cr2��SO4��3��H2SO4��H2O��H2O2����֪�÷�Ӧ��H2O2ֻ�������¹���:H2O2��O2����Ԫ�ػ��ϼ����ߣ�������������ԭ��������������ԭ��Ӧ���ٽ�ԭ��ͻ��ϼ�����ԭ����ʽ����ΪK2Cr2O7+3H2O2+4H2SO4=Cr2(SO4)3+7H2O+3O2��+K2SO4��
�ٸ÷�Ӧ�У�������ΪK2Cr2O7����������ΪO2��
���õ����ŷ���ʾ������Ӧ�е���ת�Ʒ������Ŀ��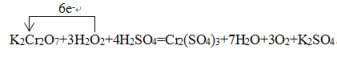 ��
��


 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������(K2FeO4)����ǿ�����ԣ���һ�����Ͷ��ˮ�������������������������£�
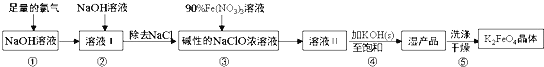
��֪��K2FeO4��ˮ��Һ��������Ӧ��4FeO42+10H2O4Fe(OH)3+8OH��+3O2��������˵������ȷ����( )
A.���������������뻹ԭ�������ʵ���֮��Ϊ3:2
B.��������Na2FeO4ת��Ϊʪ��Ʒ����ΪK2FeO4�ܽ�ȸ�С
C.�������е�ϴ�Ӽ�����CH3COOK�������������
D.����90%Fe(NO3)3��Һ����IJ�������������ƿ���ձ�����Ͳ������������ͷ�ιܵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��糧�ղ����ķ�ú�ҵ���Ҫ�ɷ�ΪSiO2��Al2O3��FeO��Fe2O3��MgO��TiO2�ȡ��о�С���������ۺϴ������������£�
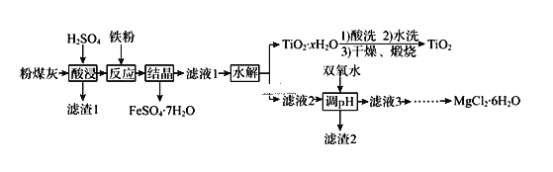
��֪���������£�Ksp[Al(OH)3] �� 8.0��10-35������Һ1������Fe2+ʣ���Fe3+��ȫ����ʱpHΪ3.7��Al3+��ȫ����ʱpHΪ5.0��Fe2+��ȫ����ʱpHΪ9.7��
��1��Ϊ����߷�ú�ҽ������ʣ����������ʱ�ɲ�ȡ�Ĵ�ʩ��_________����д���֣���
��2�������ж�TiO2xH2O�����Ѿ�ϴ�Ӹɾ�________________________________��
��3������˫��ˮ��Ŀ����_____________________________________������2�еijɷֱַ�ΪAl(OH)3��_______________��Al(OH)3������ȫʱ(��Һ������Ũ��С��10-5mol/L)����Һ�е�c(OH-)Ϊ________��
��4����MgCl26H2O�Ƴ���ˮMgCl2ʱӦע��__________________________________��
��5��ijͬѧ��ʯīΪ�����缫�����MgCl2��Һ����ȡ����þ������Ϊ���ܷ��óɹ���_______���û�ѧ����ʽ����ԭ��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼÿһ�����ʾ�йص�һ�ַ�Ӧ���������,����A��CΪ��ɫ����,D������ʹ�����ǵ�ľ����ȼ,X��һ�ֳ�������ʽ�Ρ�
����д���пհ�:
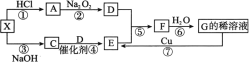
(1) ����X��������___________;����C����ķ�����___________��
(2) ��Ӧ�ܵĻ�ѧ����ʽΪ___________��
(3) ��Ӧ�����������뻹ԭ��������֮��Ϊ___________;��Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ___________��
(4) д��X������NaOH��Һ��Ӧ�����ӷ���ʽ:____________��
(5) �ڱ�״����,��һ���������ƿ��F��D�Ļ������[V(F)��V(D)=4��1]��Ȼ������Ȫʵ��,����ƿ��������Һ�����ʵ����ʵ���Ũ��Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���û�����������������þ�����ʵ��̿�(��MnO2��MnCO3)���������̣�ʵ����ģ�������������£�
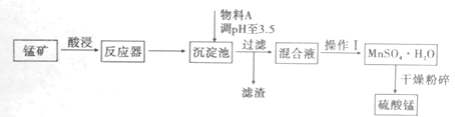
��֪����������ȫ����ʱ��pHֵ���£�Fe3+��3.5��Fe2+��9.5��Mn2+��10.8��Mg2+��11.6��
��1����Ӧ���з���������ԭ��Ӧ�����ӷ���ʽ��________��
��2���Ӿ���Ч�濼�ǣ�����A������________��
��3�������̺�����þ���ܽ����������ͼ��ʾ��
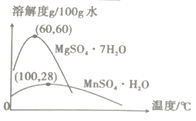
��������������Ҫ����Ϊ________��
��4��MnSO4��H2O��1150�������·ֽ⣬������Mn2O4�������ˮ���ڸ������������̾���ֽⷴӦ�Ļ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
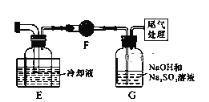
����Ŀ����ͼ��ʾ��װ���У�A���ö���������ȡ��������װ�ã�C��DΪ���徻��װ�ã�C��װ�б���ʳ��ˮ��D��װ��Ũ����)��E��Ӳ�ʲ�����װ��ϸ��˿����FΪ����Ŀչ��ƿ���ձ�G��װ������������Һ��
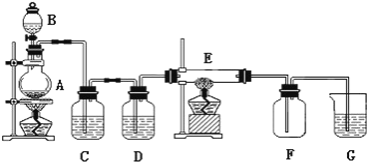
��ش��������⣺
��1������B��������________________��
��2��ʵ�����������Ļ�ѧ����ʽ��________________��
��3��Cװ�õ�������________________��Dװ�õ�������________________��
��4��E�з�����ѧ��Ӧ�ķ���ʽΪ��________________��
��5���ձ�G�з�����Ӧ�Ļ�ѧ����ʽΪ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��25 ��ʱ������H2A��HA-��A2-����ˮ��Һ��ϵ�У�H2A��HA-��A2- �����и�����ռ�����ʵ�����������������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����

A. �ں�H2A��HA-��A2- ����Һ�У���������NaOH���壬����HA-��һ������
B. �������ʵ�����NaHA��Na2A���������ˮ��������Һ������HA-��=����A2-��
C. NaHA��Һ�У�HA-��ˮ����������HA-�ĵ�������
D. �ں�H2A��HA-��A2-����Һ�У���c(H2A)+2c(A2-)+c(OH-)=c(H+)��������H2A��������HA-����һ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����λ�ڵڢ�A�壬���Ȼ�������ɫ��ǿ�ҳ�ζ�ķ���Һ������壬���ڻ�ѧΣ��Ʒ����Ҫ���ڰ뵼���IJ���Դ���л��ϳɵĴ�������Ӧ���ڸߴ����л���(�磺������B2H6)����ȡ��ijͬѧ�������Cl2�͵���B�Ʊ����Ȼ���(BCl3)��װ��ʾ��ͼ���������ϣ���BCl3�е�12.5�棬�۵㣭107.3�棬�׳��⣻��2B��6HCl![]() 2BCl3����3H2��������������Խ��������ƣ�����������������Һ��Ӧ����ش��������⣺
2BCl3����3H2��������������Խ��������ƣ�����������������Һ��Ӧ����ش��������⣺
(1)װ��A�ж�����������Ũ�����ڼ��������·�����Ӧ��ȡ�������÷�Ӧ�����ӷ���ʽ��______________________________��ϴ��ƿG����Na2SO3��Һ��ȥCl2��Ӧ�Ļ�ѧ����ʽ��____________________________��
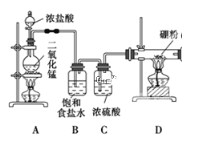
(2)Eװ�õ�������_________________________�������ȥBװ�ã����ܵ��µĺ���ǣ�_____________________________________________________________��
(3)д��Dװ���з�����Ӧ�Ļ�ѧ����ʽ��______________________________��ʵ���п���һ��ʢװ__________(��Լ�����)�ĸ���ܴ���F+Gװ�ã�ʹʵ�����㡣
(4)BCl3��ˮ���ҷ�Ӧ��������(H3BO3)�Ͱ�����д���÷�Ӧ����ʽ��____________��
(5)Ϊ��˳�����ʵ�飬��ȷ�IJ�����____________(����ֱ��)��
���ȵ�ȼA���ƾ��ƣ����ȼD���ƾ��� ���ȵ�ȼD���ƾ��ƣ����ȼA���ƾ��Ƣ�ͬʱ��ȼA��D���ƾ���
(6)����ά���ڽ���˿�ϳ������ε�����γɵ�����ά��ͨ���ڳ��ȵ���˿���淢�����µķ�Ӧ��3H2��2BCl3 ��2B��6HCl����Ҫ����֤�ƵõIJ�Ʒ���Ƿ���ۣ�Ӧȡ������Ʒ���Թ��У��μ�Ũ____________(���ѧʽ)��Һ���۲��Ƿ������ݲ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʱ����2L�ܱ�������ͨ��һ����������X��Y��������Ӧ��3X(g)+Y(g)![]() 2Z(g) ��H��0��Y�����ʵ���n(Y)��ʱ��t�仯��������ͼ��ʾ������˵����ȷ���ǣ� ��
2Z(g) ��H��0��Y�����ʵ���n(Y)��ʱ��t�仯��������ͼ��ʾ������˵����ȷ���ǣ� ��
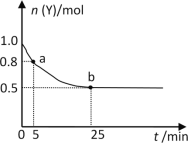
A.�÷�ӦΪ���ȷ�Ӧ
B.0~5min�ڣ���X��ʾ�Ļ�ѧ��Ӧ������0.02mol��L-1��min-1
C.b��ʱ�÷�Ӧ�ﵽ������ȣ���Ӧֹͣ
D.25minʱ��c(Z)=0.5mol��L-1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com