
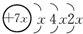 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C�������������δ�ɶԵ��ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺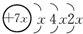 ��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�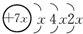 ��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�
��K���������2�����ӣ�����x=2����Aԭ�Ӻ�����14�����ӣ�ΪSiԪ�أ�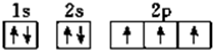 �����������Ų�Ҫ��ѭ����ԭ�������ع���
�����������Ų�Ҫ��ѭ����ԭ�������ع��� �����������أ�
�����������أ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| W | ԭ�ӵ��Ӳ���������������� |
| X | ԭ�Ӻ���L����s�ܼ��ĵ�������Ϊp�ܼ��ϵĵ���������һ�� |
| Y | Ԫ�ص�ԭ�Ӱ뾶�ڶ����������ϡ�������⣩ |
| Z | ԭ�Ӻ���p�ܼ��ϵĵ�����������s�ܼ��ϵĵ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | 1 | 2 | 3 |
| ����NaOH��Һ�������mL�� | 20.05 | 20.00 | 19.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���к͵ζ�ʵ���е���ƿʹ��ǰӦ���ô�װҺϴ�� |
| B����25 mL�ζ��ܽ��еζ�ʵ��ʱ������ij��Һ���Ϊ21.70 mL |
| C����������ƽ�������ϸ���һ�Žྻ��ֽƬֱ�ӳ���NaOH���� |
| D���ù㷺pH��ֽ���ij��Һ��pHΪ2.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
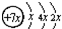 ��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺
��B��ͬ���ڵ�һ��������С��Ԫ�أ�C��������������ɵ����ӣ�E����Χ�����Ų�ʽΪ3d64s2���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
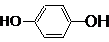
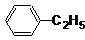
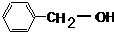
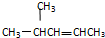
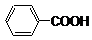
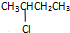
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A����Ӧ��ʼ��10s����Z��ʾ�ķ�Ӧ����Ϊ0.158mol/��L?s�� |
| B����Ӧ��ʼ��10s��X�����ʵ���Ũ�ȼ�����0.79mol/L |
| C����Ӧ��ʼ��10sʱ��Yת����0.79mol |
| D����Ӧ�Ļ�ѧ����ʽΪ��X��g��+Y��g��?Z��g�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��� | �������������/mL | �������Ƶ����/mL | ��Һ��PH |
| �� | 33.00 | 0.00 | 8 |
| �� | 33.00 | X | 7 |
| �� | 33.00 | 33.00 | 6 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com