
����Ŀ��1, 2-����������Ʊ�ԭ����CH3CH2OH![]() CH2��CH2��+H2O��CH2��CH2+Br2��BrCH2��CH2Br��ij����С������ͼ��ʾ��װ���Ʊ�1, 2-�������顣
CH2��CH2��+H2O��CH2��CH2+Br2��BrCH2��CH2Br��ij����С������ͼ��ʾ��װ���Ʊ�1, 2-�������顣
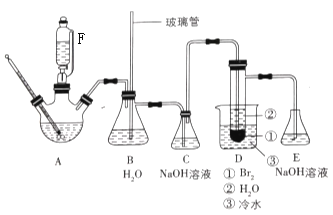
�ش��������⣺
��1����װ��������и�ʵ��ǰ������еIJ�����___________��
��2��װ��B��������____________��
��3������ƿ�ڼ���һ�������Ҵ�-Ũ������Һ��������ɰ�������ɰ��Ŀ����_____������F��������________��
��4��װ��C�ڷ�������Ҫ��Ӧ�����ӷ���ʽΪ_________��
��5������������ƿǰ���Ƚ�C��D���Ӵ��Ͽ����ٽ�������ƿ��ʯ�����ϼ��ȣ����¶�����Լ120��ʱ������C��D����Ѹ�ٽ�A��Ӧ�¶�������160��180�棬��F�������μ��Ҵ�-Ũ������Һ��������ϩ������ȵ�ͨ��װ��3.20mLҺ��![]() ��3mLˮ��D���Թܣ�ֱ����Ӧ������
��3mLˮ��D���Թܣ�ֱ����Ӧ������
�ٽ�C��D���Ӵ��Ͽ���ԭ����_____________________��
���жϷ�Ӧ������������____________________
��6������Ʒ�����Һ©�����ֱ���a. ˮϴ�ӣ�b. ����������Һϴ�ӣ�c. ���ˣ�d. ����ˮ�Ȼ��Ƹ��e. �����ռ�129��133����֣����õ�7.896g 1, 2-�������顣
�ٴ�Ʒ�ᴿ�IJ�����______������ţ�����1, 2-��������IJ���Ϊ________��
��7�����в����У����ᵼ�²�����ʽ��͵���______������ȷ�𰸵ı�ţ�
a. ��ϩͨ����ˮʱ����̫�� b. װ��C�е�NaOH��Һ��ˮ����
c. ȥ��װ��D�ձ��е�ˮ d.ʵ��ʱû��Eװ�� e. D�е��Թ��ﲻ��ˮ
���𰸡����װ�õ������� ��ȫƿ������������ƽ��װ�������ѹǿ ��ֹ���� ��Һ©������ѹ©���� SO2+2OH-==SO32-+H2O �����������ӷ� D���Թ�����ˮ��ȫ��ɫ abade 70% d
��������
��1���漰�����Ʊ������ʵ�ʵ�飬��װ�����������������Լ��飻
��2��װ��B�еIJ������������ͨ������ƽ��װ�������ѹǿ��
��3���Ҵ�-Ũ������Һ����ʱҪ��ֹ���У�
��4��װ��C����A�����ɵ������������ʣ�
��5���� �������ӷ��ش�
����D�е���ˮ��ɫ����������ȫ��Ӧ��
��6���ٴ�Ʒ1, 2-����������ᴿ�����ǣ�ˮϴ������������Һϴ�ӡ�ˮϴ������ˮ�Ȼ��Ƹ��������
��1, 2-��������IJ���=ʵ�ʲ��������۲�����100%��
��7��D�е���ӷ����屻�����������Ķ��ܽ���1, 2-��������IJ��ʡ�
��1����ʵ�����Ʊ���ϩ���壬������ϩ����ˮ��Ӧ��ȡ1, 2-�������飬������װ�����������������Լ��飻
��2��װ��B�еIJ������������ͨ������ƽ��װ�������ѹǿ����ȫƿ�����ã�
��3���Ҵ�-Ũ������Һ����ʱҪ��ֹ���У�����ƿ�ڼ���һ�������Ҵ�-Ũ������Һ��������ɰ�������ɰ��Ŀ���Ƿ�ֹ���У�����װ��ͼ������F�Ǻ�ѹ©����
��4��Ũ���������ˮ�ԡ�ǿ�����ԣ�Aװ���п��ܺ�����������SO2��CO2��SO2����ˮ�ܷ�Ӧ����ʵ�飬ʵ��װ��C����A�����ɵ�SO2��CO2���壬��Ҫ��Ӧ���ӷ���ʽ��SO2+2OH-==SO32-+H2O��
��5�������ӷ�����C��D���Ӵ��Ͽ����Լ���D���������ӷ���
����D�е���ˮ��ɫ����������ȫ��Ӧ�����Է�Ӧ������������D���Թ�����ˮ��ȫ��ɫ��
��6���ٴ�Ʒ1��2-����������ᴿ�����ǣ�ˮϴ������������Һϴ�ӡ�ˮϴ������ˮ�Ȼ��Ƹ��������Ʒ�ᴿ�IJ�����abade��
��3.20mLҺ������ʵ�����3.20mL��![]() ��160g/mol=0.06mol��1��2-�����������۲�����0.06mol��188g/mol=11.28g��1��2-��������IJ���=ʵ�ʲ��������۲�����100%=7.896g��11.28g��100%=70%��
��160g/mol=0.06mol��1��2-�����������۲�����0.06mol��188g/mol=11.28g��1��2-��������IJ���=ʵ�ʲ��������۲�����100%=7.896g��11.28g��100%=70%��
��7��a.��ϩͨ����ˮʱ����̫�죬��ӷ��ӿ죬�ᵼ�²�����ʽ��ͣ�b.װ��C�е�NaOH��Һ��ˮ���棬��ɶ����������巴Ӧ���ᵼ�²�����ʽ��ͣ�c.ȥ��װ��D�ձ��е�ˮ����ӷ��ӿ죬�ᵼ�²�����ʽ��ͣ�d.Eװ�õ�������β��������ʵ��ʱû��Eװ�ã��Բ�����Ӱ�죻e.��ϩ���巴Ӧʱ���ȣ�D�е��Թ��ﲻ����ˮ������ӷ����ᵼ�²�����ʽ��ͣ���ѡd��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ܱ������н��еĿ��淴Ӧ��aA��g����bB��g��![]() cC��g���ڲ�ͬ�¶���T1��T2����ѹǿ��p1��p2���£����������B����������w��B���뷴Ӧʱ����t���Ĺ�ϵ��ͼ��ʾ�������ж���ȷ����
cC��g���ڲ�ͬ�¶���T1��T2����ѹǿ��p1��p2���£����������B����������w��B���뷴Ӧʱ����t���Ĺ�ϵ��ͼ��ʾ�������ж���ȷ����

A��T1<T2��p1<p2��a��b>c������ӦΪ���ȷ�Ӧ
B��T1>T2��p1<p2��a��b<c������ӦΪ���ȷ�Ӧ
C��T1<T2��p1>p2��a��b<c������ӦΪ���ȷ�Ӧ
D��T1>T2��p1>p2��a��b>c������ӦΪ���ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����п�ͼ��ʾ������ת����ϵ�У������ճ������г����Ľ������ҡ��������dz��������嵥�ʡ�����B ������C �������������İ��̣�D �Ǻ�ˮ��Ũ����ߵ���(���ַ�Ӧ��������P�ܼ�ˮ����ȥ)��

��ش��������⣺
(1) B��E �ĵ���ʽΪ______________________��__________________��
(2)��ҵ�ϳ��õ�ⱥ�� D ��Һ���Ʊ�������д���˷�Ӧ�Ļ�ѧ����ʽ��_______����������Ϊ___________________________��
(3)A �� E ���������·�Ӧ�Ļ�ѧ����ʽ______________________________��
(4)�Һͱ���Ӧ�Ļ�ѧ����ʽ______________________________________��
(5)B �������ڴ��������ܷ�����Ӧ��д���仯ѧ��Ӧ����ʽ��_____��
(6)��̬�� A �� C �������ͷֱ�����_________��__________����̬�� A ����ʱ��Ҫ�˷�������������Ϊ___________����C ����ˮ��Ҫ�˷���������Ϊ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��֤��±��Ԫ�صķǽ�����ǿ����ijС������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ��)��
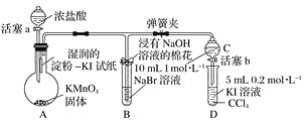
ʵ����̣�
��.���ɼУ�����a���μ�Ũ���ᡣ
��.��B��C�е���Һ����Ϊ��ɫʱ���н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ�غ�ɫʱ���رջ���a��
��.����
(1)��֤������������ǿ�ڵ��ʵ��������________________________________________��
(2)B����Һ������Ӧ�����ӷ���ʽ��____________________________________________��
(3)Ϊ��֤���������ǿ�ڵ⣬���� �� �IJ�����������____________________________��
(4)���̢�ʵ���Ŀ����________________________________________________________��
(5)�ȡ��塢�ⵥ�ʵ�������������ԭ��ͬ����Ԫ�ش��ϵ��£�ԭ�Ӱ뾶��________���õ���������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����С��Լ����͡��Թ��е����ʡ�������ɡ�ʵ��Ŀ�ġ�����

ʵ��Ŀ�� | �Լ� | �Թ��е����� | |
A | �ǻ��Ա����Ļ�����Ӱ�� | ������ˮ | �ٱ��ڱ�����Һ |
B | ���Ա���������Ӱ�� | ����KMnO4��Һ | �ٱ��ڼױ� |
C | ��������û��̼̼˫�� | Br2��CCl4��Һ | �ٱ��ڼ�ϩ |
D | ̼������Աȱ���ǿ | ʯ����Һ | �ٱ�����Һ��̼����Һ |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȼ������һ��CH4����Ҫ��Դ�����ÿ�ȼ���Ʊ��ļ��������Ƴ�����ȼ�ϵ�أ�������Ϊ���ƶ��ķ��糧����ԴԴ���ϵĶ���磬����˵����������

A.a�缫ͨ��������壬������
B.ÿͨ��1mol CH4����8mol e��ͨ�����ӽ���Ĥ
C.b�缫�ĵ缫��ӦʽΪ��O2��4e����4H����2H2O
D.�����缫�������ɶ�ṹ����ߵ缫��ӦЧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͨ������£�NCl3��һ����״Һ�壬����ӿռ乹����NH3���ƣ����ж�NCl3��NH3���й�������ȷ����( )
A. ������N��Cl��������CCl4������C��Cl���������
B. NCl3�����ǷǼ��Է���
C. NBr3��NCl3�ӷ�
D. �ڰ�ˮ�У���NH3��H2O�����(����������ʾ)����γ�NH3��H2O���ӣ���NH3��H2O�ĽṹʽΪ![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����BaSO4������Һ�м���������BaCl2��Һ����BaSO4����������Ksp��ʾBaSO4���ܶȻ���������ƽ�����Һ��
A.c(Ba2��)��c(SO42��)��Ksp��c(Ba2��)>c(SO42��)
B.c(Ba2��)��c(SO42��)��(Ksp)1/2
C.c(Ba2��)��c(SO42��)>Ksp��c(Ba2��)��c(SO42��)
D.c(Ba2��)��c(SO42��)��Ksp��c(Ba2��)<c(SO42��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��д��SO3���ӵĿռ乹����____����____���ӣ����������������Ǽ��������������ĵȵ�����Ļ�ѧʽ��һ��������______ (д��һ��)�����ǵ�����ԭ�Ӳ��õ��ӻ���ʽ����_______��
��2����ȩ(H2C��O)��Ni�������¼���ɵü״�(CH3OH)���״�������Cԭ�ӵ��ӻ���ʽΪ__���״������ڵ�O��C��H����___����������������������������ȩ�����ڵ�O��C��H���ǣ��״���������ˮ������Ҫԭ����_____��
��3����֪�ߵ�����������ʽ����ѧʽ�ֱ�ΪH5IO6��HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ����H5IO6_____��������������������������HIO4��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com