
����Ŀ����һ��������Fe��Fe2O3��CuO�Ļ�Ϸ�ĩ���뵽100mL 4.4mol/L �������У���ַ�Ӧ��ַ�Ӧ�����ɱ�״���µ�����896mL�����ˣ�������ϴ�ӡ���������������Ϊ1.28g����Һ�е�����ֻ��FeCl2��HCl����Һ��ˮϡ�͵�320mLʱ�������Ũ��Ϊ0.25mol/L��
��ش�
(1)��Ӧ�������������ʵ���Ϊ_______ mol��
(2)����ԭ������������ʵ�����(д���������)��________
���𰸡�0.04 5.6g
��������
(1)����n=![]() �����������������ʵ�����
�����������������ʵ�����
(2)����������ᷴӦ��������ʣ�࣬�������û��Fe��ֻ��Cu����Һ�к���ʣ���HCl��FeCl2������Clԭ���غ�n��(HCl)=nʣ��(HCl)+2n(FeCl2)���ݴ˼���n(FeCl2)����ԭ��FeΪx mol��Fe2O3Ϊy mol������FeԪ���غ�͵���ת���غ��з��̼�����
(1)���ɱ�״���µ�����896mL�������ʵ���Ϊ![]() =0.04mol���ʴ�Ϊ��0.04��
=0.04mol���ʴ�Ϊ��0.04��
(2)����������ᷴӦ��������ʣ�࣬�������û��Fe��ֻ��Cu����Һ�к���ʣ���HCl��FeCl2������Clԭ���غ㣺n��(HCl)=nʣ��(HCl)+2n(FeCl2)����0.1L��4.4mol/L=0.32L��0.25mol/L+2n(FeCl2)�����n(FeCl2)=0.18mol��n(CuO)=n(Cu)=![]() =0.02mol����ԭ��FeΪx mol��Fe2O3Ϊy mol����FeԪ���غ㣬�ɵã�x+2y=0.18�����ݵ���ת���غ㣬�ɵã�2x=2y+2��0.02+2��0.04���������̣���ã�x=0.1��y=0.04��ԭ������е�����������56g/mol��0.1mol=5.6g���ʴ�Ϊ��5.6g��
=0.02mol����ԭ��FeΪx mol��Fe2O3Ϊy mol����FeԪ���غ㣬�ɵã�x+2y=0.18�����ݵ���ת���غ㣬�ɵã�2x=2y+2��0.02+2��0.04���������̣���ã�x=0.1��y=0.04��ԭ������е�����������56g/mol��0.1mol=5.6g���ʴ�Ϊ��5.6g��


 ���ſ����ϵ�д�
���ſ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����0.3mol����̬����ȼ�������飨B2H6����������ȼ�գ����ɹ�̬�����������Һ̬ˮ���ų�649.5kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ______________������֪��H2O��g��=H2O��l������H2����44.0kJ/mol����11.2L����״������������ȫȼ��������̬ˮʱ�ų���������_____________kJ��
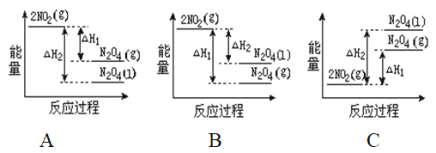
��2����֪��2NO2��g��![]() N2O4��g����H1 2NO2��g��
N2O4��g����H1 2NO2��g��![]() N2O4��l����H2
N2O4��l����H2
���������仯ʾ��ͼ�У���ȷ���ǣ�ѡ����ĸ��_____________��
��3�����ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�������㡣
��֪��C��s��ʯī����O2��g����CO2��g�� ��H1����393.5 kJ��mol��1
2H2��g����O2��g����2H2O��l�� ��H2����571.6 kJ��mol��1
2C2H2��g����5O2��g����4CO2��g����2H2O��l�� ��H3����2 599 kJ��mol��1
���ݸ�˹���ɣ�����298 Kʱ��C��s��ʯī����H2��g������1 mol C2H2��g����Ӧ���ʱ䣨�г��ļ���ʽ����___________________________��
��4���״���һ�����͵���������ȼ�ϣ���ҵ�Ͽ�ͨ��CO��H2�������Ʊ��״����壨�ṹ��ʽΪCH3OH���� ��֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | H��O |
����/kJ��mol��1 | 348 | 413 | 436 | 358 | 1072 | 463 |
��֪CO�е�C��O֮��Ϊ�������ӣ���ҵ�Ʊ��״����Ȼ�ѧ����ʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���±���Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ��밴Ҫ����д���пհף�
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
2 | �� | �� | �� | �� | |||
3 | �� | �� | �� | �� |
(1)��Ԫ�آ٢ڢݢޢߢ��Ӧ������������ˮ�����У�������ǿ�Ļ����������ʽ�ǣ�_________��
(2)д��Ԫ�آڵ�����⻯��Ļ�ѧʽ____________��
(3)�ܢݢޢ�����Ԫ�صļ����Ӱ뾶�Ӵ�С����____________(�����ӷ��ű�ʾ)��
(4)д��Ԫ�آ������������Ԫ�آݵ�����������ˮ���ﷴӦ�����ӷ���ʽ______________________________________��
(5)д��Ԫ�آ۵ij����⻯�����������������ˮ���ﷴӦ�Ļ�ѧ����ʽ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CaC2����ľ����ṹ��NaCl���������(��ͼ��ʾ)����CaC2���������������ε�![]() ���ڣ�ʹ������һ���������������й���CaC2�����˵������ȷ����
���ڣ�ʹ������һ���������������й���CaC2�����˵������ȷ����

A.1��Ca2+��Χ��������ҵȾ����![]() ��ĿΪ4
��ĿΪ4
B.�þ����е���������F2�ǵȵ�����
C.6.4 g CaC2�����������0.1 mol
D.��ÿ��Ca2+��������������Ca2+����12��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ͭ��Ũ���ᷴӦ��ʵ��װ��ͼ��

(1)�����ٵ�������_____________��
(2)ʵ������У��Թܢ��з���������Ϊ____________________________________��
(3)�Թܢ���ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ _______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£���̼��![]() ��
��![]() ��
��![]() ������
������![]() ������Һ���Ũ��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������
������Һ���Ũ��NaOH��Һ��Ӧ�����Һ�У����ǵ����ʵ�������![]() ij�����ʵ���Ũ�������������ʵ���Ũ�Ⱥͱ�ֵ
ij�����ʵ���Ũ�������������ʵ���Ũ�Ⱥͱ�ֵ![]() ����ҺpH�Ĺ�ϵ��ͼ��ʾ�������й�˵���������
����ҺpH�Ĺ�ϵ��ͼ��ʾ�������й�˵���������![]()
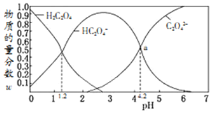
A.��![]() ����Һ�м�NaOH��Һ��pH������
����Һ�м�NaOH��Һ��pH������![]() �Ĺ�����ˮ�ĵ����һֱ����
�Ĺ�����ˮ�ĵ����һֱ����
B.![]() ʱ����Һ��c
ʱ����Һ��c![]()
![]()
![]()
C.![]() ������ĵڶ�������ƽ�ⳣ��Ϊ
������ĵڶ�������ƽ�ⳣ��Ϊ![]() ����
����![]()
D.��![]() ��ͬ���ʵ���Ũ��
��ͬ���ʵ���Ũ��![]() ��
��![]() ��������Һ�������ϣ������ͼa����ʾ���Һ
��������Һ�������ϣ������ͼa����ʾ���Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ��![]() �ں��������н��У��ﵽƽ��״̬�ı�־��( )
�ں��������н��У��ﵽƽ��״̬�ı�־��( )
�ٵ�λʱ��������![]() ��ͬʱ����
��ͬʱ����![]()
�ڵ�λʱ��������![]() ��ͬʱ����
��ͬʱ����![]()
����![]() ��
��![]() ��
��![]() �����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ2��2��1��״̬
�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����֮��Ϊ2��2��1��״̬
�ܻ���������ɫ���ٸı��״̬
�ݻ��������ܶȲ��ٸı��״̬
��������ƽ����Է����������ٸı��״̬
A.�٢ܢ�B.�ڢۢ�C.�٢ۢ�D.�٢ڢۢܢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ѡ��[��ѧ��ѡ��3:���ʽṹ������] (15����X��Y��Z��W��R��QΪǰ30��Ԫ�أ���ԭ��������������X������Ԫ����ԭ�Ӱ뾶��С�ģ�Y�������ܼ�����ÿ���ܼ��ϵĵ�������ȣ�Zԭ�ӵ���������ͬ����Ԫ������࣬W��Zͬ���ڣ���һ�����ܱ�Z�ĵͣ�R��Yͬһ���壬Q�������ֻ��һ�����ӣ��������Ӳ���Ӿ����ڱ���״̬����ش��������⣺
��1��R��������Ų�ʽΪ__________________��
��2��X��Y��Z��W�γɵ��л���YW(ZX2��2��Y��Z���ӻ�������ͷֱ�Ϊ__________��ZW3-���ӵ����幹����__________��
��3��Y��R�����������ķе�ϸߵ���_____________���ѧʽ����ԭ����_________________��
��4����Q���ʵķ�ĩ���뵽ZX3��Ũ��Һ�У���ͨ��W2����ַ�Ӧ����Һ������ɫ���÷�Ӧ�����ӷ���ʽΪ______________________________________��
��5��W��Na��һ�����ӻ�����ľ����ṹ��ͼ�������ӻ�����Ϊ____________���ѧʽ����Na+����λ��Ϊ_____________����һ����������Χ���������������Ϊ���㹹�ɵļ�����Ϊ__________����֪�þ������ܶ�Ϊ��g��cm-3�������ӵ�����ΪNA�������������W���Ӽ����Ϊ nm(�ú�����NA�ļ���ʽ��ʾ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NH3���������з�ȼ�ϵ���ǵ�ǰ���е�һ���ȵ㡣ʹ�õĵ������Һ��2mol��L1��KOH��Һ������ܷ�ӦΪ��4NH3+3O2��2N2+6H2O���õ�ظ����ĵ缫��ӦʽΪ____________________��ÿ����3.4g NH3ת�Ƶĵ�����ĿΪ_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com