
���� ��1�����������Һ����ǿ�����ԣ��ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�
��2������KMnO4��Һ�Ļ�ԭ����ΪMnSO4��+4�۵�������Ϊ+6�ۣ�������������ӣ����ݵ��ӵ�ʧ�غ㡢�����غ��Լ�����غ�����ƽ��
��3��KMnO4��Һ����ɫ������ᷴӦ����ɫ��ȥ�����Ե������һ��KMnO4��Һ����ɫ����ȥ��˵���ζ����յ㣻���ݹ�ϵʽ��2MnO4-��5HSO3-����NaHSO3�ߵ����ʵ�����������Ȼ���������������
��4������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��1�����������Һ����ǿ�����ԣ��ܸ�ʴ��ʽ�ζ��ܵ���Ƥ�ܣ�����ѡ����ʽ�ζ�����ȡ���������Һ��
�ʴ�Ϊ����ʽ�ζ��ܣ�
��2������KMnO4��Һ�Ļ�ԭ����ΪMnSO4��+4�۵�������Ϊ+6�ۣ�������������ӣ���Ӧ�����ӷ���ʽΪ��H++2MnO4-+5HSO3-=2Mn2++5SO42-+3H2O��
�ʴ�Ϊ��H++2MnO4-+5HSO3-=2Mn2++5SO42-+3H2O��
��3��KMnO4��Һ����ɫ������ᷴӦ����ɫ��ȥ���ζ��յ������Ϊ���������һ��KMnO4��Һ����ɫ����ȥ��
�ʴ�Ϊ���������һ��KMnO4��Һ����ɫ����ȥ��
��ȷ��ȡWgNaHSO3��������ˮ���500mL��Һ��ȡ25.00mL������ƿ�У���KMnO4��Һ�ζ����յ㣬����KMnO4��ҺVmL��
2MnO4-��5HSO3-
2 5
0.1000mol/L��V��10-3L n��HSO3-��
��ã�n��HSO3-��=$\frac{5}{2}$��V��10-4mol����25.00mL��Һ��NaHSO3�����ʵ���Ϊ=$\frac{5}{2}$��V��10-4mol������500mL��Һ��NaHSO3�����ʵ���Ϊ=$\frac{5}{2}$��V��10-4mol$\frac{500}{25}$=5��V��10-3mol��NaHSO3���������Ϊ5��V��10-3mol��104g/mol=5.2��V��10-1g������Ϊ$\frac{5.2��V��1{0}^{-1}g}{Wg}$��100%=$\frac{13V}{25W}$��100%��
�ʴ�Ϊ��$\frac{13V}{25W}$��100%��
��4��A��δ�ñ�Ũ�ȵ�����KMnO4��Һ��ϴ�ζ��ܣ���Һ��Ũ��ƫС�����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫ��A��ȷ��
B���ζ�ǰ��ƿδ�������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c���������䣬��B����
C���ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ��������ݣ����V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫ��C��ȷ��
D����С�Ľ���������KMnO4��Һ������ƿ�⣬���V������ƫ����c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫ��D��ȷ��
E���۲����ʱ���ζ�ǰ���ӣ��ζ����ӣ����V������ƫС������c�����⣩=$\frac{c����ע����V������}{V�����⣩}$������c������ƫС����E����
��ѡACD��
���� ���������ʺ����ⶨΪ����������������ԭ�ζ��������������Լ����㣬�ѶȲ���ע��ζ�ԭ�������գ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
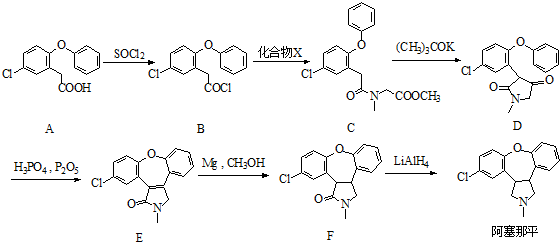
 ��
�� �����������ȡ��������ͬһ�������ϣ�
�����������ȡ��������ͬһ�������ϣ�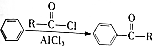 ��RΪ����������������֪ʶ����������Ϣ��д����ClCH2CH2CH2Cl��
��RΪ����������������֪ʶ����������Ϣ��д����ClCH2CH2CH2Cl��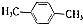 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ�����ѡ�����ϳ�·������ͼʾ����ͼ��ʾ��
�ĺϳ�·������ͼ�����Լ�����ѡ�����ϳ�·������ͼʾ����ͼ��ʾ��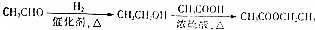
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼���� | B�� | ����������Һ | C�� | �������� | D�� | KI��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaCl2��NaNO3��NH4Cl | B�� | K2CO3��HCl��CaCl2 | ||
| C�� | Na2CO3��HNO3��HCl | D�� | K2CO3��KCl��H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
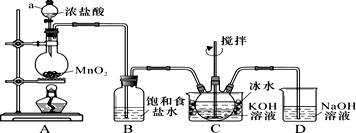
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���뾶��Na+��Al3+��S2-��Cl- | B�� | �е㣺F2��Cl2��Br2��I2 | ||
| C�� | ���ԣ�LiOH��NaOH��KOH��RbOH | D�� | �۵㣺Li��Na��K��Rb |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������̼�ͳ���ʯ��ˮ��Ӧ | B�� | ʵ������˫��ˮ��ȡ���� | ||
| C�� | ̼���������ȷֽ� | D�� | ����ͭ��ϡ���ᷴӦ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com