
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ��������ͼ��ʾ��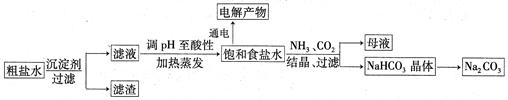
��1��Ϊ��ȥ�����е�Ca2+��Mg2+��SO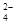 ���õ�������NaCl���壬����������Լ���
���õ�������NaCl���壬����������Լ���
| A��������NaOH��Һ�� | B��������Na2CO3��Һ�� | C����������� | D��������BaCl2��Һ�� |
 ��OH-���ı�ֵ��_________��
��OH-���ı�ֵ��_________�� ��1�� adbc��dabc (2��) ���˺������ᾧ (2��)
��2��OH-��CO32- (2��) H+ + OH- = H2O (1��) CO32- + 2H+ = CO2��+ H2O (1��)
��3��13 (2��)
��4��NH3 (1��) ��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
��5���� CO32- + H2O HCO3- + OH-������Һ�ɳ����� (2��) �� 1010��1 (2��)
���������������1�����ӹ����У���ʱΪ�ܽ�����ȫ����ȥ�����ó����Լ������Լӹ��������������Լ�����Ҫ�ܱ������Լ��������������˽����ʵ�鲽���Ŀ�ģ����ܷ������˳���������NaOH��Һ��Ŀ���dz�ȥMg2�������������BaCl2��Һ��Ϊ�˳�ȥSO42-�����������Na2CO3��Һ��Ϊ�˳�ȥCa2���������Ba2����Ȼ����й��ˣ���ȥMg(OH)2��BaSO4��CaCO3��BaCO3�������Ȼ������������ᣬ��ȥ�����Na2CO3�͵�����Һ��pH��������������ȷ��ʵ�����˳��Ϊadbc��dabc����ȱ�ٵIJ��������ǹ��˺������ᾧ��
��2��������NaOH��Na2CO3��Ӧ�����ӷ���ʽΪ��H+ + OH- = H2O��CO32- + 2H+ = CO2��+ H2O��
��3�������Ȼ�����Һ��Ӧ�����ӷ���ʽΪ��2Cl?+2H2O  2OH?+H2��+Cl2�������ܷ�Ӧ�������Ϲ��ռ���0.448L��0.02 mol����ʱ�������ɵ���������������0.01mol�����������������Ƶ����ʵ�����0.02mol�������������Ƶ�Ũ��C=0.02mol��0.2L=0.1mol?L?1,����pH=13��
2OH?+H2��+Cl2�������ܷ�Ӧ�������Ϲ��ռ���0.448L��0.02 mol����ʱ�������ɵ���������������0.01mol�����������������Ƶ����ʵ�����0.02mol�������������Ƶ�Ũ��C=0.02mol��0.2L=0.1mol?L?1,����pH=13��
��4�������NaHCO3������ĸҺ��NH4Cl�����������ʯ�ң�NH4Cl��Ca(OH)2��Ӧ��õĿ���ѭ��ʹ�õ�����ΪNH3��
��5����Na2CO3Ϊǿ�������Σ�ˮ���Լ��ԣ����ӷ���ʽΪ��CO32- + H2O 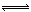 HCO3- + OH-��������Һ�ɳ����ۡ�
HCO3- + OH-��������Һ�ɳ����ۡ�
�ڷ�Ӧǰ����Һ��ˮ�������c��OH-��=1.0��10-14��10-11=1.0��10-3mol/L����Ӧ����ҺΪpH=13��ˮ�������c��OH-��=1.0��10-13mol/L�����Ա�ֵΪ1.0��10-3mol?L?1��1.0��10-13mol?L?1=1010��1��
���㣺���⿼����ε��ᴿ�����ӷ���ʽ����д�����ԭ�������Ӻ����ʵ��жϡ�����Ũ�Ⱥ�pH�ļ��㡣


 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ش��������⡣
��1������ʱ��FeCl3��Һ��pH 7�����������������������ʵ��������FeCl3��Һʱ����FeCl3���������ڽ�Ũ��������Һ�У�Ȼ����������ˮϡ�͵������Ũ�ȣ�ԭ���� ����FeCl3��Һ���ɡ����գ��˹��������漰���Ļ�ѧ����ʽ�� ��
��2��25��ʱ����0.1 mol��L��1��ˮ�м�������NH4Cl���壬�������ܽ�����ҺpH��С����Ҫԭ���� ��
��3��ij��Һ��ֻ����OH����H+��Na+��CH3COO���������ӡ�
������Һ��ֻ�ܽ���һ�����ʣ������ʵ������� ������Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ ��
������Һ���������ӵĴ�С˳��Ϊc��Na+����c��OH������c��CH3COO������c��H+��������Һ�����ʵĻ�ѧʽΪ ��
������Һ��c��Na+����c��CH3COO�����������Һ�� ������ԡ��������ԡ��������ԡ���������Һ�������ȵ�ϡNaOH��CH3COOH��Һ��϶��ɣ�����ǰc��NaOH�� c��CH3COOH�������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E������Һ�ֱ���NaOH��NH3·H2O��CH3COOH��HCl��NH4HSO4�е�һ�֡������½�������ʵ�飺
�ٽ�1 L pH��3��A��Һ�ֱ���0.001mol·L-1 xL B��Һ��0.001mol·L-1 yL D��Һ��ַ�Ӧ�����ԣ�x��y��С��ϵΪ��y��x����Ũ�Ⱦ�Ϊ0.1mol·L-1A��E��Һ��pH��A��E����Ũ�Ⱦ�Ϊ0.1mol·L-1C��D��Һ�������ϣ���Һ�����ԡ�
�ش��������⣺
��1��D�� ��Һ���ж������� ��
��2����ˮϡ��0.1 mol·L-1Bʱ����Һ������ˮ�������Ӷ���С���� (��д���)��
��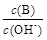 ��
��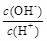 ��c(H+)��c(OH-)�ij˻� ��OH-�����ʵ���
��c(H+)��c(OH-)�ij˻� ��OH-�����ʵ���
��3��OH-Ũ����ͬ�ĵ������������ҺA��E���ֱ���п�۷�Ӧ����������һ����Һ�д���п�ۣ��ҷų�������������ͬ��������˵����ȷ����________(��д���)��
�ٷ�Ӧ����Ҫ��ʱ��E>A �ڿ�ʼ��Ӧʱ������A>E
�۲μӷ�Ӧ��п�����ʵ���A=E �ܷ�Ӧ���̵�ƽ������E>A
��A��Һ����п��ʣ�� ��E��Һ����п��ʣ��
��4����������������ʵ���Ũ��B��C��Ϻ���Һ�������¶�(���ʲ���ֽ�)��ҺpH���¶ȱ仯����ͼ�е�_________����(��д���) ��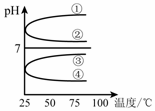
��5�������£���0.01mol·L-1 C��Һ�еμ�0.01mol·L-1 D��Һ�����ԣ��õ�����Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ���¶��£������ᡢ���ᡢ����������Һ���밴Ҫ��ش��������⣺
��1��������������ʵ���Ũ����ͬʱ�����������c(H+)������__________��
��2�������ߵ��������c(H+)��ͬʱ������������ʵ���Ũ����С����________��
��3��pH��ͬ�������ͬ�Ĵ�����Һ������ֱ���������п��Ӧ���տ�ʼ��Ӧʱ�ķ�Ӧ���ʵĴ�С��ϵΪ______(���ȡ����ߡ����ȡ�)����ͬ״���²��������������������______��pH��ͬ�Ĵ�����Һ�����ᣬ�ֱ�������ˮϡ����ԭ�����m����n����ϡ�ͺ�����ҺpH����ͬ����m��n�Ĺ�ϵ�ǣ�m n(���������������������)
��4��ͬ�����ͬ���ʵ���Ũ�ȵĴ�������������ᣬ�ֱ��к�ͬŨ�ȵ�NaOH��Һ������NaOH�������С��ϵΪ (���ȡ����ߡ����ȡ�)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2����Pb(OH)����Pb(OH)2��Pb(OH)3-��Pb(OH)42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ����ͼ��ʾ�� 
(1)Pb(NO3)2��Һ�У� ________2(�>����������<��)��������Һ�е����Ȼ����Һ��
________2(�>����������<��)��������Һ�е����Ȼ����Һ�� ���ӣ����ܵ�ԭ����________________________________��
���ӣ����ܵ�ԭ����________________________________��
(2)��Pb(NO3)2��Һ�е���ϡNaOH��Һ��pH��8ʱ��Һ�д��ڵ�������(Na������)��__________��pH��9ʱ��Ҫ��Ӧ�����ӷ���ʽΪ_______________________��
(3)ij�������Ʊ���һ��������Ǧ��������Чȥ��ˮ�еĺ���Ǧ��ʵ�������±���
| ���� | Pb2�� | Ca2�� | Fe3�� | Mn2�� | Cl�� |
| ����ǰŨ��/(mg��L��1) | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ��/(mg��L��1) | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
 E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )
E2Pb(s)��2H������Ǧ�������pH��ΧΪ( )�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ʽ̼��ͭ��һ����;�㷺�Ļ���ԭ�ϡ���ҵ�Ͽ������Կ�ʴ��Һ����Ҫ�ɷ���Cu2+��Fe2+��Fe3+��H +��Cl-���Ʊ������Ʊ��������£�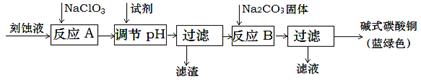
Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ҫ�Ĺ�ҵԭ�ϡ�
��1������ʱ�������ʵ����������������ڴ���������ǡ����ȫ��Ӧ���Ƶ����ơ���Ӧ�Ļ�ѧ����ʽΪ__________________��
��2����ͬѧ��ijNa2S��Ʒ����Na2CO3��Na2SO4���ʣ���Һ�м�������BaS��Һ��������ɫ���������ˣ��������м��˹������ᣬ������ȫ�ܽ⡣�ɴ˵ó����ۣ���ͬ�¶��£�Ksp(BaCO3)<Ksp(BaSO4)��
�ٳ���������������ӷ���ʽ��__________________��
�ڽ�������ʵ�����ж�Ksp(BaCO3)��Ksp(BaSO4)�Ĵ�С��ϵ��������______��
��3������Na2SΪ��������п�ҿ��Ƶ�ZnS��п�Ҿ�ϡ�����ȡ�����ý�ȡҺ��Zn2+��Cd2+��Al3+��Fe2+, Fe3+�ȣ��ɸý�ȡҺ�Ʊ�ZnS�Ĺ�����������ͼ��ʾ��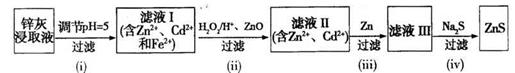
�ٲ��裨i�����������к�����Ԫ�ص�����Ϊ______���ѧʽ����
�ڲ��裨ii������ZnO������Ϊ____________��
�۲��裨iii���еõ�Cd���ʵ����ӷ���ʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D������������ˮ����ȫ���룬��������������±���
| ������ | Na+��Al3+��Ba2+��H+��NH4+ |
| ������ | SO42-��OH-��CO32-��Cl- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
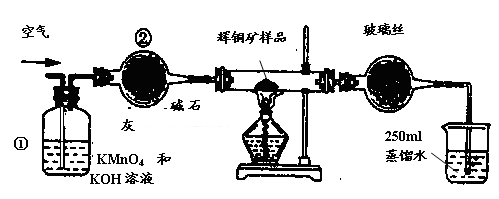
��ҵ��Ϊ�˲ⶨ��ͭ����Ҫ�ɷ���Cu2S����Cu2S�������������������ͼװ�á�ʵ��ʱ�����²��������
| A������ȫ��������ʹ���Ϊ��ͼװ�ã������װ�õ������ԡ� |
| B����ȡ��ϸ�Ļ�ͭ����Ʒ1.000g�� |
| C���������õ���ƷС�ĵط���Ӳ�ʲ������С� |
| D����ÿ����1L�����ʹ�������� |
| �ζ� ���� | ������Һ�� ���/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com