
����Ŀ��H2C2O4���Ҷ��������������ᣬ��������Ȼ���ֲ���С���֪�����²������ƽ�ⳣ��K1=5.0��10-2��K2=5.4��10-5��̼��ĵ���ƽ�ⳣ��K1=4.5��10-7��K2=4.7��10-11��Ksp (CaC2O4)=2.3��10-9��Ksp(CaCO3)=2.5��10-9���ش�����������
��1��д��������Һ�д��ڵ���Ҫ���뷴Ӧ����ʽ_____________________________________��
��2�����ڽ������벤�˻��ʳ�ã�������ʧ�����һ�����ʯ������������______________________��
��3��25�������ʵ���Ũ�Ⱦ�Ϊ0.1mol/L ��Na2C2O4��Һ��pH��Na2CO3��ҺpH_________������������ С���������������������������Һ�������Ϻμ�CaCl2��Һ����C2O42- ������ȫʱ��CO32-�Ƿ������ȫ_______________��������������������
��4��д��NaHC2O4��Һ��ˮ�ⷴӦ�����ӷ���ʽ________________________________�����㳣���¸÷�Ӧ��ˮ��ƽ�ⳣ��Ϊ_____________________��NaHC2O4��Һ��pH____7 �����������������=����
��5����ʢ�б��Ͳ�����Һ���Թ��е��뼸�������ữ��KMnO4��Һ����������Һ���Ϻ�ɫ��ȥ��д���÷�Ӧ�����ӷ���ʽ________________________________________��
��6�����ʵ��֤����������Ա�̼��ǿ________________________________________��
���𰸡� H2C2O4![]() HC2O4-+H+ �����и������벤���в��ᷴӦ�������ܵIJ���ƣ��������������� С �� HC2O4-+ H2O
HC2O4-+H+ �����и������벤���в��ᷴӦ�������ܵIJ���ƣ��������������� С �� HC2O4-+ H2O![]() H2C2O4 + OH- 2.0��10-13 < 2MnO42- + 6H+ + 5H2C2O4 =2Mn2+ + 10CO2��+8H2O ��NaHCO3��Һ�м��������Һ����������������˵����������Ա�̼��ǿ
H2C2O4 + OH- 2.0��10-13 < 2MnO42- + 6H+ + 5H2C2O4 =2Mn2+ + 10CO2��+8H2O ��NaHCO3��Һ�м��������Һ����������������˵����������Ա�̼��ǿ
����������1��H2C2O4Ϊ��Ԫ���ᣬ��Ҫ������һ�����룬���뷽��ʽΪ��H2C2O4![]() HC2O4-+H+��
HC2O4-+H+��
��2�������м����˵����CaSO4�������к��в��ᣬCa2+����ᷴӦ�������ܵIJ���ƣ��������������ա���Ϊ�������и������벤���в��ᷴӦ�������ܵIJ���ƣ��������������ա�
��3��HC2O4-�ĵ���ƽ�ⳣ��Ϊ5.4��10-5��HCO3-�ĵ���ƽ�ⳣ��Ϊ4.7��10-11������HC2O4-�����Դ���HCO3-�����ԣ�����C2O42-��ˮ��̶�С��CO32-��ˮ��̶ȣ�����ˮ�ⶼ�ʼ��ԣ�������ͬŨ�ȵ�Na2C2O4��Һ��pH��Na2CO3��ҺpHС��C2O42-������ȫʱ��C2O42-��Ũ��Ϊ1��10-5mol/L����ʱc(Ca2+)=2.3��10-9/1��10-5=2.3��10-4(mol/L)����c(CO32-)=2.5��10-9��(2.3��10-4mol /L)=1.1��10-5mol /L>1.0��10-5mol /L��Ca2+δ������ȫ��
��4��NaHC2O4��Һ��ˮ�ⷴӦ�����ӷ���ʽΪ��HC2O4-+ H2O![]() H2C2O4 + OH-����ˮ�ⷴӦ��ƽ�ⳣ��K=c(H2C2O4)��c(OH-)/c(HC2O4-)������1/K= c(HC2O4-)/ c(H2C2O4)��c(OH-)= c(HC2O4-)��c(H +)/ c(H2C2O4)��c(OH-)��c(H+)= K1(H2C2O4)/Kw=5.0��10-2/1.0��10-14=5��1012����K=2.0��10-13��HC2O4-��ˮ�ⳣ��K=2.0��10-13������ƽ�ⳣ��K=5.4��10-5��ˮ��ƽ�ⳣ��С�ڵ���ƽ�ⳣ��������̶ȴ���ˮ��̶ȣ�����NaHC2O4��Һ�����ԣ�pH<7��
H2C2O4 + OH-����ˮ�ⷴӦ��ƽ�ⳣ��K=c(H2C2O4)��c(OH-)/c(HC2O4-)������1/K= c(HC2O4-)/ c(H2C2O4)��c(OH-)= c(HC2O4-)��c(H +)/ c(H2C2O4)��c(OH-)��c(H+)= K1(H2C2O4)/Kw=5.0��10-2/1.0��10-14=5��1012����K=2.0��10-13��HC2O4-��ˮ�ⳣ��K=2.0��10-13������ƽ�ⳣ��K=5.4��10-5��ˮ��ƽ�ⳣ��С�ڵ���ƽ�ⳣ��������̶ȴ���ˮ��̶ȣ�����NaHC2O4��Һ�����ԣ�pH<7��
��5��������л�ԭ�������Ը�����ؾ��������ԣ����߷���������ԭ��Ӧ�����ӷ���ʽΪ: 2MnO42- + 6H+ + 5H2C2O4 =2Mn2+ + 10CO2��+8H2O��
��6������ǿ���������ԭ����ֻҪ����������̼�ἴ��֤����������Դ���̼������ԣ��ʿ�����NaHCO3��Һ�м��������Һ����������������˵����������Ա�̼��ǿ��


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������һ����Ҫ�Ļ�����Ʒ��ij��ȤС�����Ʊ���������ƾ���(Na2S2O3��5H2O)����������֪��
��Na2S2O3��5H2O����ɫ������,������ˮ,��ϡ��Һ��BaCl2��Һ����������ɡ�
����Na2CO3��Na2S�����Һ��ͨ��SO2���Ƶ�Na2S2O3��
��BaSO3������ˮ,������ϡHCl��
ʵ��װ����ͼ��ʾ(ʡ�Լг�װ��)
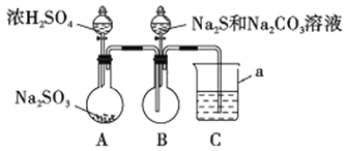
��1������a��������_________;C�е��Լ�������______ (ѡ��������ĸ���)��
A��ϡH2SO4 B������KMnO4��Һ C������NaHSO3��Һ D��NaOH��Һ
��2����ʵ��װ�������ȱ��,��Ľ�������_________________________��
��3��д��B�з�Ӧ�����ӷ���ʽ_________________________________________��
��4��A��B�з�Ӧ���,�ڲ�װ��ǰ��Ӧ��������Ⱦ�������ж������ȥ,���õķ����;��������________________________________________��
��5���÷����ò�Ʒ�г���������Na2SO3��Na2SO4��Ϊ��֤��Ʒ�к���Na2SO3��Na2SO4,��С�����������ʵ�鷽��,�뽫��������������(�����Լ���ϡHNO3��ϡH2SO4��ϡHCl������ˮ��ѡ��)
ȡ������Ʒ���ϡ��Һ��������BaCl2��Һ,�а�ɫ�������ɣ�_______________��������δ��ȫ�ܽ�,���д̼�����ζ���������,���ȷ����Ʒ�к���Na2SO3��Na2SO4��
��6���ⶨ��Ʒ���ȣ�ȷ��ȡWg��Ʒ,����������ˮ�ܽ�,�Ե�����ָʾ��,��0.1000mol/L��ı���Һ�ζ���(��Ӧԭ��Ϊ��2S2O32-+I2=S4O62-+2I-)
�ٵζ����յ�ʱ,��Һ��ɫ�ı仯��_______________________��
�ڵζ���¼�������±���
�ζ�ǰ����/mL | �ζ������/mL | |
��һ�� | 0.10 | 16.12 |
�ڶ��� | 1.10 | 17.08 |
������ | 1.45 | 19.45 |
���Ĵ� | 0.00 | 16.00 |
�۲�Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ϩ�ӳɲ������
A.CH3CH3B.CH3CHCl2C.CH3CH2OHD.CH3CH2Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Һ�����ʵ���Ũ�ȵļ�����ȷ����
A. ��״���£�a L NH3����1000 gˮ�У��õ�����Һ�ܶ�Ϊb gcm-3����������Һ����仯�������Һ�����ʵ���Ũ��Ϊ![]() molL-1
molL-1
B. ��100 mL 1.5 molL-1��NaCl��Һ��200 mL 2.5 molL-1��NaCl��Һ��ϣ�������Һ����仯�����õ���Һ�����ʵ���Ũ��Ϊ2 molL-1
C. V L Fe2��SO4��3��Һ�к�Fe3+m g����Һ��c��SO42-����![]() molL-1
molL-1
D. ij�¶�ʱ������NaCl��Һ���ܶ�Ϊ�� gcm-3�����ʵ���Ũ��Ϊcmol/L������Һ��NaCl����������Ϊ![]() ��100%
��100%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ����̽��SO2��Fe(NO3)3��Һ��Ӧ��ԭ����װ������ͼ��ʵ����Yװ�ò�����ɫ����������˵������ȷ����

A. �μ�Ũ����֮ǰӦ���еIJ����Ǵ��ɼУ�ͨ��һ��ʱ��N2
B. Y�в����İ�ɫ������BaSO4��BaSO3
C. ������ɫ������ԭ�������������������SO2��NO3- ��Ӧ������SO42-
D. ����Fe(NO3)3����FeCl3��Y��Ҳ�ܲ�����ɫ������˵��Fe3+Ҳ�ܽ�SO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ����Ҫ���л�ԭ�ϣ��ڴ����������£�CO��H2��Ӧ�����ɼ״� (CH3OH) ������CH4����Ӧ������
��Ӧ��CO(g)+2H2(g)![]() CH3OH(g) ��H1=-90.0kJ/mol
CH3OH(g) ��H1=-90.0kJ/mol
�� Ӧ��CO(g)+3H2(g)![]() CH4(g) + H2O(g) ��H2
CH4(g) + H2O(g) ��H2
��Ӧ�� CH4(g)+2H2O(g)![]() CO2(g)+ 4H2(g) ��H3=+125.0 kJ/mol
CO2(g)+ 4H2(g) ��H3=+125.0 kJ/mol
��Ӧ��CO(g)+ H2O(g)![]() CO2(g) + H2(g) ��H4=-25.0 kJ /mol
CO2(g) + H2(g) ��H4=-25.0 kJ /mol
K1��K2��K3��K4�ֱ��ʾ��Ӧ����������������ƽ�ⳣ����
�ش�����������
��1����Ӧ����ƽ�ⳣ���ı���ʽΪK2=______________��K2��K3��K4�Ĺ�ϵΪK2=______________����H2=____________kJ/mol��
��2��ͼ1������ȷ��ʾ��Ӧ����ƽ�ⳣ��(lgK1) ���¶ȱ仯������Ϊ______________����������ĸ�������ж�����Ϊ______________________________________________________________��

��3�����º��ݵ������£����������˵����Ӧ���ﵽƽ��״̬����__________________��
A.2v�� (H2)=v��(CH3OH) B.���������ܶȲ��ٸı�
C.��������ƽ��Ħ���������ٸı� D.��������ѹǿ���ٸı�
��4��Ϊ̽����ͬ������CO��H2����CH3OH��ѡ����Ч����ijʵ���ҿ���CO��H2�ij�ʼͶ�ϱ�Ϊ1��3����ʵ�飬�õ�����������
T/K | ʱ��/min | �������� | �״��ĺ���(%) |
450 | 10 | CuO-ZnO | 78 |
450 | 10 | CuO-ZnO-ZrO2 | 88 |
450 | 10 | ZnO-ZrO2 | 46 |

���ɱ�1��֪����Ӧ������Ѵ���Ϊ______________��ͼ2��a��b��c��d�ĵ��Ǹ��¶���COƽ��ת���ʵ���_________________________________��
�����������COת��ΪCH3OH��ƽ��ת���ʵĴ�ʩ��_________________��
A.ʹ�ô���CuO-ZnO-ZrO2 B.�ʵ����ͷ�Ӧ�¶�
C.����CO��H2�ij�ʼͶ�ϱ� D.�����£��ٳ���a molCO��3a mol H2
��5����֪1000������ӦCO(g)+ H2O(g)![]() CO2(g) + H2(g) K4=1.0�����¶��£���ijʱ����ϵ��CO��H2O��CO2��H2��Ũ�ȷֱ�Ϊ3molL-1��1molL-1��4molL-1��2molL-1�����ʱ������Ӧ��v��(CO)_______v��(CO) �����������������=�����ﵽƽ��ʱc(CO)=___________ molL-1��
CO2(g) + H2(g) K4=1.0�����¶��£���ijʱ����ϵ��CO��H2O��CO2��H2��Ũ�ȷֱ�Ϊ3molL-1��1molL-1��4molL-1��2molL-1�����ʱ������Ӧ��v��(CO)_______v��(CO) �����������������=�����ﵽƽ��ʱc(CO)=___________ molL-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2 molCaCl2�ܽ���ˮ���2 L��Һ��������Һ���ʵ���Ũ����( )
A. 0.5 mol/L B. 1 mol/L C. 2 mol/L D. 4 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
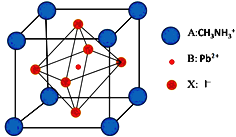
����Ŀ�������ѿ�̫���ܵ���ǽ����������о��ȵ㣬�߱�������ࡢ����Ӧ�á�����ɱ��ͺ�Ч�ʸߵ������ŵ㣬����һ�ָ��ѿ�̫���ܵ�ز��ϵľ�����ͼ���ش��������⣺
��1��Ǧ��Ǧ�ε���ɫ��ӦΪ��ɫ�������й�ԭ��������������ȷ����_________(����ĸ)��
a.���Ӵӻ�̬ԾǨ���ϸߵļ���̬ b.���Ӵӽϸߵļ���̬ԾǨ����̬
c.��ɫ��Ӧ�Ĺ����������չ��� d.��ɫ��Ӧ�Ĺ������ڷ������
��2��̼ԭ�Ӽ۲���ӵĹ������ʽ�������Ų�ͼ��Ϊ_________����̬Pbԭ�Ӻ�������Ų������ռ���ܼ��ĵ���������ͼ��״Ϊ___________��
��3��CH3NH3+�к��л�ѧ����������________������ĸ��ţ���Nԭ�ӵ��ӻ���ʽΪ______����CH3NH3+��Ϊ�ȵ�����ķ���Ϊ_________
a.���Լ� b. �Ǽ��Լ� c.��λ�� d. ���Ӽ� e.�Ҽ� f.�м�
��4��NH4+��H��N��H�ļ��DZ�NH3��H ��N��H�ļ��Ǵ��ԭ����__________��NH3��ˮ������ͭ�����γɵĻ������������ӳ����������İ�����ṹ(����ͼ)���û��������ʱ����ʧȥˮ�����ԭ�ӽṹ�Ƕȼ��Է�����__________��
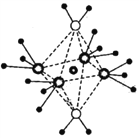
��5����I- ���ڵ�I- ����Ϊ__________��X��������ʵ���þ����������ܶ�Ϊa g��cm-3�����ı߳�Ϊ____________pm�������ʵ����ԭ������ΪM��NA��ʾ�����ӵ�������ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. ��ϩ������ԭ�˹�ƽ��
B. �����ǡ������ͺͲ�˿һ�������¶��ܷ���ˮ�ⷴӦ
C. ���������е�����������ñ���Na2CO3��Һ��ȥ
D. C4H4���� ��CH��C-CH=CH2����ͬ���칹��
��CH��C-CH=CH2����ͬ���칹��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com