
��Ϥ��ʹ����ѧ��ѧʵ���г�����������;���ǻ�ѧѧϰ�Ļ���Ҫ���Իش��������⡣
(1)������a.��Һ©����b���Լ�ƿ��c������ƿ��d���ζ��ܡ�e������ƿ��f����Ͳ��g��������ƽ�У����С�0���̶ȵ���________(�����)��
(2)��ĥɰ�������Ӳ��������ܷ��Ե�һ�ִ������գ������������У�û���õ���ĥɰ�����մ�������________(�����)��
a���Թܡ� b����Һ©���� c�����ιܵ��Լ�ƿ(��ƿ)
d������ƿ��e����ʽ�ζ��ܡ�f����ʽ�ζ���
(3)��װ�üס��ҡ������齺�����һ��װ��(�������Ѽ��)����������ȡ���ռ�NH3��HCl���壬�ɹ�ѡ���Һ���Լ��У�Ũ���ᡢŨ���ᡢŨ��ˮ�������Լ�Ϊ��ɫʯ����Һ���Իش��������⣺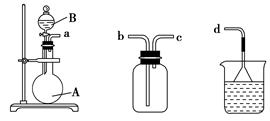
�������ס��� �����ҡ����� ����
������ȡij����Ĺ����У����е�ʯ����Һ��죬����ƿ�е��Լ�A���Һ©���е��Լ�B��Ϊ��ɫҺ�壬���Լ�AΪ________����ȡ������������B��������________��________��
ͨ��������ɫʯ����Һ���������˵�����������Ѽ�������ʯ����Һ����������ƿ����Ӧ����Ĺ����Լ�AΪ________��
�����������ռ���������������װ�õ�˳���ǣ�a�D��____�D��_____�D��d(��ӿڴ���)��
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����й�ʵ�������ȷ����
| A���ù����Ȼ�狀�����������ȡ�������������Թܴ�����̨��ȡ����������ϴ�� |
| B���ñ���ȡ��ˮ�е���ʱ������ı���Һ�ӷ�Һ©���¿ڷų� |
| C���������Ƶ�����ʵ��ʱ��ʣ����ƷŻ�ԭ�Լ�ƿ |
| D������ڵ�NaOHϡ��Һ�е���FeCl3������Һ�����Ʊ�Fe��OH��3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����������ʵķ�����ȷ����(����)
| A���������������ڶ���̼�� |
| B��ˮ���������ڴ��������IJ���ƿ�� |
| C�������������ھƾ��� |
| D��������������ú���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����йػ�ѧʵ���˵������ȷ����
| A���ձ����������Թܡ���ƿ�������þƾ���ֱ�Ӽ��� |
| B��������Ӧ����ȩ������Cu(OH)2��Ӧ��ʵ������ȡ��ϩ��������ˮԡ���� |
| C����ȡ����������ʱ�������������ſ������ռ� |
| D��ʯ�͵ķ���ʵ������ȡ��Ȳ����ȡ����ˮ��Ҫ�õ�����װ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣���ͼ��A��B��C��D�dz�������ͨ�����Լ�ƿ����������Լ��������д���ʺ�ʢ�ŵ��Լ�ƿ����������ڣ�
a��Ũ���� b��̼������Һ c����Ƭ d��Ũ���� e������������Һ f����������
| A | B | C | D |
 |  |  |  |
| �� �� | �� �� | �� �� | �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��18mol/L����������100 mL 1.0 mol/L�����ᣬ��ʵ�������У�A��100mL��Ͳ��B��������ƽ��C����������D��50 mL����ƿ��E��10 mL��Ͳ��F����ͷ�ιܣ�G��50 mL�ձ���H��100 mL����ƿ��
��1��ʵ��ʱѡ�õ�������________������������ţ�
��2��������ƿ��ʹ�÷����У����в�������ȷ���ǣ� ��
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵĵ�������ƿ�У���������ˮ���ӽ��̶���2��3cm�������õιܵμ�����ˮ���̶���
D��������Һʱ�����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������������ˮ���ӽ��̶���2��3cm�������õιܵμ�����ˮ���̶���
E���Ǻ�ƿ������ʳָ��סƿ��������һֻ����סƿ�ף�������ƿ��ת��ҡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ϣʱ�������Ĵ������������Ի�������˼������в��ij�����Ϊ����ѧ��̽��С�齫һ����������·������õ���70%Cu��25%Al��4%Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�����·�ߣ�
�ش��������⣺
�ŵڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ ���õ�����1����Ҫ�ɷ�Ϊ ��
�Ƶڢڲ�����H2O2�������� ��ʹ��H2O2���ŵ��� ������pH��Ŀ����ʹ ���ɳ�����
���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ����� ��
��������2��ȡAl2(SO4)3��18H2O��̽��С����������ַ�����
�������ַ����У� ���������У�ԭ���� ��
��ԭ�������ʽǶȿ��ǣ� ������������
��̽��С���õζ����ⶨCuSO4��5H2O(Mr=250)������ȡa g �������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol/L EDTA(H2Y2��)����Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�Cu2++ H2Y2����CuY2��+ 2H+
д������CuSO4��5H2O���������ı���ʽ�أ� ��
���в����ᵼ��CuSO4��5H2O�����ⶨ���ƫ�ߵ��� ��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ظ�����ǹ�ҵ������ʵ���ҵ���Ҫ����������ҵ�ϳ��ø�������Ҫ�ɷ�ΪFeO ? Cr2O3)Ϊԭ��������ʵ����ģ�ҵ���ø�������K2Cr2O7����Ҫ�������£��漰����Ҫ��Ӧ�ǣ�
6FeO��Cr2O3+24NaOH+7KClO3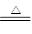 12Na2CrO4+3Fe2O3+7KCl+12H2O
12Na2CrO4+3Fe2O3+7KCl+12H2O
��ش��������⣺
��1���ڷ�Ӧ�����У���Na2CrO4���ɣ�ͬʱFe2O3ת��ΪNaFeO2������SiO2��Ai2O3�봿�Ӧת��Ϊ�������Σ�д����������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��________________��
��2) NaFeO2��ǿ��ˮ�⣬�ڲ��������ɳ�������ȥ��д���÷�Ӧ�Ļ�ѧ����ʽ��_______________��
��3����Ҫ���������۵�Ŀ�ģ�________________________��
��4���������У��ữʱ��CrO42-ת��ΪCr2O72-��д��ƽ��ת�������ӷ���ʽ��___________��
��5����ȡ�ظ��������2. 5000g���250mL��Һ��ȡ��25.00mL�ڵ���ƿ�У�����10mL2mol/LH2SO4�������⻯�أ����Ļ�ԭ����ΪCr3+)�����ڰ���5min��Ȼ�����100mLˮ������3mL����ָʾ������0.1200mol/LNa2S2O3����Һ�ζ���I2+2S2O32��=2I��+S4O62���������մﵽ�ζ��յ㹲��ȥNa2S2O3����Һ40.00mL�������ò�Ʒ�ظ���صĴ���________________ (�������������������ʲ����뷴Ӧ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ҩƷ��װ�ú������������Ӧʵ�����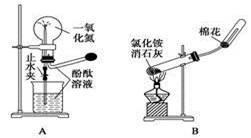
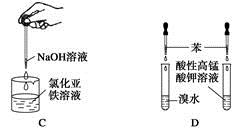
| A����Ȫʵ�� |
| B��ʵ������ȡ���ռ����� |
| C���Ʊ����������� |
| D����֤�����Ƿ���̼̼˫�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com