
����Ŀ��ȡ3.40 gֻ���ǻ��������������ŵ�Һ̬���Ͷ�Ԫ��������5.00 L���У���ȼʹ����ȫȼ�ա���Ӧ�������������0.56 L�������徭CaO���գ�����ּ���2.80 L������������ڱ�״���²ⶨ����
��1��3.40 g����C��H��O���ʵ����ֱ�ΪC_______mol,H________mol,O________mol���ô���C��H��O ��ԭ����֮��Ϊ____________��
��2�������ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ?__________����ԭ����________________________��
��3��������ô�������һ���ǻ�����һ��±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ__________________��
������
��1��0.125 0.30 0.100 5��12��4
��2������ ��Ϊ��ʵ��ʽ��Hԭ�Ӹ����Ѵﱥ��
��3��C��CH2OH��4
��������
�����������1����������֪���徭����CaO���գ��������2.80 L���������ΪCO2���������3.40 g����C�����ʵ���Ϊ��n(C)=n(CO2)= 2.80L��22.4L/mol=0.125mol������CO2����O2���������CO2�������ҲΪ2.80 L������ΪҺ̬����5.00 L O2ȼ�գ��������0.56 L���ʻ���0.56 L O2��������ˮ���������ر�ע����ǣ����ɵ�H2O�л��в�����ԭ�������ڴ�����˲��ܸ���0.56 L O2������H2O������Ҫ���ݷ�Ӧǰ���������������㴼��H�����ʵ������μӷ�Ӧ��V(O2)=0.56 L+2.80 L=3.36L��O2�����ʵ���Ϊ��0.15 mol����O2������Ϊm(O2)=0.15 mol��32 g/mol=4.80 g,m(H2O)=3.40g+4.80g-0.125��44 g=2.70 g�����ʵ�����0.15mol�����3.40 g����n(H)=2��=0.300mol����3.40g������ԭ�������ʵ�����![]() ������n(C)��n(H)��n(O)=0.125 mol��0.300 mol��0.100mol=5��12��4��
������n(C)��n(H)��n(O)=0.125 mol��0.300 mol��0.100mol=5��12��4��
��2�� ��Ϊ��ʵ��ʽ��Hԭ�Ӹ����Ѵﱥ���������������ʽΪC5H12O4�������Ƿ���ʽ��
��3��һ±����ṹֻ��1�֣��������к���һ����ԭ�ӣ�����������ṹ��ʽΪC(CH2OH)4



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ��ʵ���ҳ�����ͼ��ʾװ����ȡ����������������ش��������⣺
��1���Թ�a�������Ũ���ᡢ�����2 mL���Ҵ�3 mL��Ӧ���ȼ���________������________��������________��

��2���Թ�a�з�����Ӧ�Ļ�ѧ����ʽ��________________����Ӧ������________��ͨ�����뼸Ƭ���Ƭ����������_______________________________________________________��
��3���Թ�b�м��뱥��Na2CO3��Һ����������______________________________________��
��4����Ӧ���������Թ�b�����á��۲쵽������_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ�����������������Ҫ��;���������ܼ�����������Ҳ��������ɫ�̶�Һ�������ܼ���ʳƷ��������л��ϳ��м��塣����ϩΪԭ���Ʊ�������������ĺϳ�·�����£�
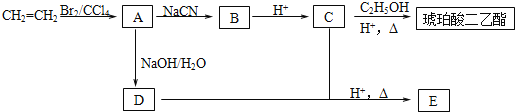
��֪����RBr+NaCN![]() RCN+NaBr
RCN+NaBr
��RCN+2H2O+H+![]() RCOOH+NH4+
RCOOH+NH4+
��ش��������⣺
��1����ϩ����A�ķ�Ӧ����Ϊ ��A�������� ��
��2��B�Ľṹ��ʽΪ ��������������Ľṹ��ʽΪ ��
��3��A![]() D�Ļ�ѧ����ʽΪ ��
D�Ļ�ѧ����ʽΪ ��
��4��EΪ��Ԫ��״�����E�к��еĹ���������Ϊ ��
��5���ܷ���������Ӧ��������̼���Ʒ�Ӧ����CO2��C��ͬ���칹���� �֡�
��6����������������������ĺϳ�·�ߣ����һ���ɱ���(CH3CH2CH2OH)�ϳ�2-������![]() �ĺϳ�·�ߣ� ��
�ĺϳ�·�ߣ� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ��[��ѧ����ѡ��5���л���ѧ����]˫��-A�Ķ�����ϩ������һ����ʹ�˼�������ڷ���ϵͳ�������ң�������������ֳ�쳣�Ļ������أ���ṹ��ʽΪ��
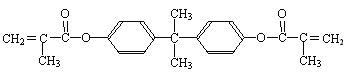
����һ��������ˮ�������˫��-A������H�������ʡ�
I����1�����й���˫��-A����������ȷ���� ����д��ĸ����
a���뱽�ӻ�Ϊͬϵ��
b�����Ժ�Na2CO3��Һ��Ӧ���ų�CO2����
c�����������е�̼ԭ�ӿ�����ͬһƽ����
d��1mol˫��-A��Ũ��ˮ��Ӧ������������Br2�����ʵ���Ϊ4 mol
��2��������������˫��-A��Ϊͬ���칹����� ����д��ĸ����
a��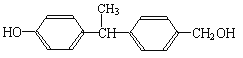
b��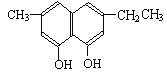
c��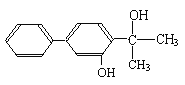
d��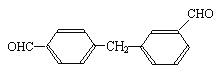
II����֪��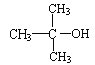 �ṹ���ƵĴ����ܱ�����Ϊȩ���ᡣ
�ṹ���ƵĴ����ܱ�����Ϊȩ���ᡣ
����H����������;���Ƶã�
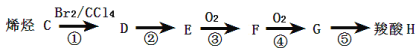
��3��C�Ľṹ��ʽΪ ��
��4��G������������������Ϊ ����Ӧ���ķ�Ӧ����Ϊ ��
��5����Ӧ���Ļ�ѧ����ʽΪ ����Ӧ���Ļ�ѧ����ʽΪ ��
��6������H��״���Ӧ��õ����������γ�һ�ָ߷��ӻ�����������������ѧ����������д���������ɴ˸߷��ӻ�����Ļ�ѧ��Ӧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ����7ƿ�л���A��B��C��D��E��F��G�����ǿ����DZ����ױ�����ϩ�����ӡ��Ҵ�����ȩ�����ᣬΪȷ��ÿƿ�ɷ֣���������ʵ�飺
��ȡ7�������л���ֱ��ˮ������ˮ�ֲ����A��D��G��
��ȡ����A��D��G�ֱ������ˮ����ֻ��D������ʹ��ˮ��ɫ�������Ͳ�Ҳ��ɫ��
��ȡ����A��G���ֱ��������KMnO4��Һ����G��ʹ����KMnO4��Һ��ɫ��
��ȡA����������ŨH2SO4��ŨHNO3�����Ⱥ���ˮ�У��п�����ζ��ɫ��״Һ�����ɣ�
��ȡ����B��C��E��F���ֱ����������Һ����ˮԡ���Ⱥ�ֻ��B����������
��ȡ����C��E��F�����ֱ��������ƣ�����H2�ų������ֱ����Na2CO3��Һ��ֻ��F������ų���
��ȡ����C��E������FeCl3��Һ��E��Һ����ɫ��
��ȡ����C��F����Ϻ����ŨH2SO4�����Ⱥ��й���ζ����״Һ�����ɡ�
��1�����ƶ�A��G����ʲô���ʣ�����ϩд����ʽ��������д�ṹ��ʽ����
A_________��B_________��C_________��D_________��E_________��F_________��G_________��
��2��д��B����������Ӧ�Ļ�ѧ����ʽ____________________________________��
��3��д��ʵ������Ӧ�Ļ�ѧ����ʽ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����Ŀ���л���A-G֮���ת����ϵ��ͼ��ʾ����Ҫ��ش��������⣺

��1��A������Ϊ______��C�����ں˴Ź�����������______�����շ壮D�Ľṹ��ʽΪ______��F���еĹ�����������______��
��2��ָ�����з�Ӧ������_____________________��
��3����Ӧ���Ļ�ѧ����ʽΪ____________________________��
��4����Ӧ���Ļ�ѧ����ʽΪ____________________________��
��5��F�ж���ͬ���칹�壬������������������ͬ���칹����___________�֡�
����FeC13��Һ������ɫ��Ӧ
�����������������ȡ�������ұ����ϵ�һ±����ֻ������
���ܷ���������Ӧ
��1mol���л������������Ʒ�Ӧ�����ɱ�״����22.4L������
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
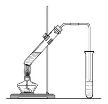
����Ŀ����ʵ��������Ҳ������ͼ��ʾ��װ����ȡ����������
�ش��������⣺
��1���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ� ��
��2��Ũ����������ǣ��� ��
�� ��
��3������̼������Һ����Ҫ������ ��
��4��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У���Ŀ��
�� ��
��5����Ҫ���Ƶõ������������������Ӧ���õ�ʵ������� ��
��6������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
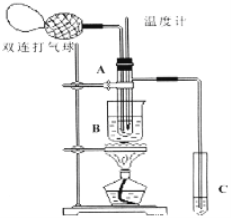
����Ŀ����ȩ������ͭ���£����Ա��������������ᡣ���ݴ�ԭ�������֤ʵ������ͼ��ʾ���Թ�A��װ��40������ȩˮ��Һ������ͭ��ĩ���Թ�C��װ����������ˮ�����ռ�����������Һ���ձ�B��װ��ijҺ��������֪��60�桫80��ʱ����˫�����������������ɷ�����ȩ��������Ӧ����������ʮ���η�Ӧ������ȫ���й����ʵķе���±���

��ش��������⣺
��1���Թ�A����60��80��ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��ע����Ӧ������ ��
��2����ͼ��ʾ��ʵ��IJ�ͬ�Σ���Ҫ�����¶ȼ����Թ�A�ڵ�λ�ã���ʵ�鿪ʼʱ�¶ȼ�ˮ�����λ��Ӧ�� ��Ŀ���� �����Թ�A�ڵ���Ҫ��Ӧ��ɺ��¶ȼ�ˮ�����λ��Ӧ�� ��Ŀ���� ��
��3���ձ�B�������� ���ձ�B��ʢװ��Һ������� �� �����ϱ���������ѡ����д�ṹ��ʽ����
��4����������Թ�C���Ƿ��в������ᣬ����������ҩƷ�н���ѡ�����һ������ʵ�鷽�����ɹ�ѡ���ҩƷ�У�pH��ֽ����ɫ��ʯ����ֽ����ɫ�Ĵ���Ǧ��ֽ��̼�����Ʒ�ĩ��ʵ��������ѡ���÷���Ϊ________ ��
��5����֪��ȩ�ܱ���ˮ������д���÷�Ӧ�Ļ�ѧ����ʽ______________________________ .
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
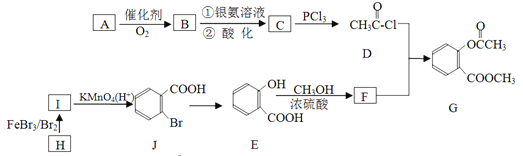
����Ŀ���л���G��һ��ҽҩ�м��壬��ͨ����ͼ��ʾ·�ߺϳɡ�A��ʯ�ͻ�������Ҫ��Ʒ�ҷ���������ԭ����ͬһƽ���ϣ�H�ķ���ʽ��C7H8��[
��֪��![]()
��ش��������⣺
��1��A������ʽ��________________________________��
��2��C��D�ķ�Ӧ������________________________________��
��3����һ��������������E���ӷ������Ӽ���ˮ����һ�ֻ�״����д���û�״�����ṹ��ʽ__________��
��4��G������������������Һ��Ӧ�Ļ�ѧ����ʽ��________________________________��
��5����������������F��ͬ���칹��(����F)����_________________����
�������Ȼ�����Һ������ɫ��Ӧ�ڿ��Է���ˮ�ⷴӦ
���к˴Ź����������շ����ٵ�ͬ���칹��Ľṹ��ʽΪ___________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com