
����Ŀ�������Ķ�
����ֽ
˫��A��Bisphenol A����ѧʽC15H16O2����ɫ��״������
![]()
˫��A ����дΪBPA����������ֽ�ϳ䵱��Ӱ������ɫȾ�ϣ�Ũ�ȸߴ�ÿ��ֽԼ20���ˡ���������ֽ�����ڷɻ�Ʊ��ATM�������ͻ�����������ƾ���Լ��������͵�СƱ������ֽ����Ӱ������ɫ��Ӧ�ǿ���ģ���ɫ����ͬ�̶ȵ����зֽ⣬�ּ���ɫ��������ɫԽ��Խdz����ֱ����Ȼ��ɫ���ּ���ȫ��ʧ�ڰ�ֽ֮�С��о������״�֤��������СƱ������ֽ������Ϳ��˫��A��BPA����ͨ���Ӵ���ճ�������ϣ�Ȼ���ֵ�Ƥ�����������գ�ʹ�����ڵ�BPA����������ߡ�


�ڿ�͵�ȳ��������Ǿ����ڽ�ʳǰʹ���ֲ���ϴ����Һ��Ȼ����ʱ�ֻ�Ӵ���СƱ����ϴ����Һ�к��ⶹޢ������������������Ҵ��ȳɷ֡��о�������ͼ�˽���һ���������������BPA�к�Ӱ�죬Ϊ��������ʵ�顣��ʵ���У����ñ�������һֻ�ֲ�����ϴ����Һ���ڻ�δ��������£�������ֱ����ס����ֽ����һֻ��δʹ������Һ���ø����������סͬ����С������ֽ��������Ʊ���������BPA������ȡ�ͷ������������ͼ��
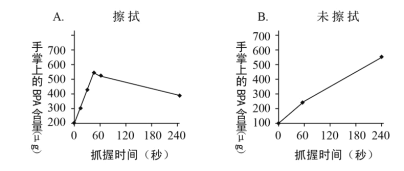
�����������ϣ��ش��������⣺
��1��˫��A����_____����л�����������һ��˫��A�����к���_____����ԭ�ӡ�
��2����СƱ���ܷ���Ϊ��Ҫ��֤�ݳ��ڱ���,��˵��ԭ��_____��
��3����������ͼ���Կ�������ʹ����ϴ����Һ��������BPA�ĺ����ı仯������_____����δʹ������Һ�ĶԱȣ� ˵����ϴ����Һ��_____�����BPA�����ա�
��4����Ȼ����˫��A�Ķ���ѧ�о����кܶ࣬������������Ķ���Ŀǰ��û��Ȩ����ȷ�еĽ��ۡ��о���Ա��Ϊ��Ӧ�ÿ�������ȫ�IJ��ϻ�����������ֽ�����������£�����Ϊ�ڿ�����Ͳ�ǰ������������_____���Է�˫��A������롣
���𰸡��л��� 2 ���ܣ�����ֽ����Ӱ������ɫ��Ӧ�ǿ���ģ���ɫ����ͬ�̶ȵ����зֽ⣬�ּ���ɫ��������ɫԽ��Խdz����ֱ����Ȼ��ɫ���ּ���ȫ��ʧ�ڰ�ֽ֮�� ��ʱ�����ӣ�BPA���������Ӻ���� ���٣���ǿ�����ӵȣ� ����ϴ��Һ�����Ӵ�СƱ
��������
����˫��A�Ļ�ѧʽ�����ʡ����ݶԱȺ��������з�����
��1������̼Ԫ�صĻ����̼���������̼���γ��⣩���л��˫��A�����л��һ��˫��A�����к���2����ԭ�ӡ�
��2����СƱ�����ܷ���Ϊ��Ҫ��֤�ݳ��ڱ��棬����ֽ����Ӱ������ɫ��Ӧ�ǿ���ģ���ɫ����ͬ�̶ȵ����зֽ⣬�ּ���ɫ��������ɫԽ��Խdz����ֱ����Ȼ��ɫ���ּ���ȫ��ʧ�ڰ�ֽ֮�С�
��3����������ͼ���Կ�������ʹ����ϴ����Һ��������BPA�ĺ����ı仯��������ʱ�����ӣ�BPA���������Ӻ���٣���δʹ������Һ�ĶԱȣ� ˵����ϴ����Һ�ܼ��٣���ǿ�����ӵȣ������BPA�����ա�
��4�����������£�ʹ����ϴ����Һ��������BPA�ĺ����ı仯��������ʱ�����ӣ�BPA���������Ӻ���٣���δʹ������Һ�ĶԱȣ� ˵����ϴ����Һ�ܼ��٣���ǿ�����ӵȣ������BPA�����ա������ڿ�����Ͳ�ǰ�����������Dz���ϴ��Һ�����Ӵ�СƱ���Է�˫��A������롣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ִ������У�����Խ��Խע����Ԫ�ص���ȡ����Ԫ�ض����彡����������Ҫ�����ã��±���ij���г����۵�һ�����ӵ�ʳ������װ���ϵIJ���˵����
���� | �Ȼ��ơ�����أ�KIO3�� |
������ | ��20mg��30mg��/kg |
������ | 18���� |
ʳ�÷��� | ��ʱ������ |
���淽�� | �ܹ⡢���ȡ��۷䡢���� |
��ش��������⣺
��1����ʳ�÷���������ָ�Ͽ��Ʋ����أ�KIO3���Ļ�ѧ����֮һ��_____��
��2���������أ�KIO3���У���Ԫ�ء���Ԫ�ء���Ԫ�ص�������_____��
��3���������أ�KIO3���У���Ԫ�ص����������Ƕ��٣�_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ȥ���������л��е�����������ѡ�õ��Լ�������ȷ���ǣ� ��
ѡ�� | ����������Ϊ���ʣ� | ѡ�õ��Լ� |
A |
| �������͵�����������Һ |
B |
| ����������ͭ��ĩ |
C |
| ���ȵ����� |
D |
| �������͵�����������Һ |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס������������ֲ���ͼ��ʾ����ͷ����ˮ�����������a��ȡˮ����⣬ˮ�к�������NaOH��Na2SO4����c��ȡˮ����⣬pH��7��ˮ��ֻ��������NaCl����b��ˮ�к��е�һ�������ǣ�������
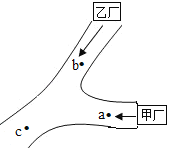
A.MgSO4 NaClB.H2SO4 Ba��NO3��2
C.BaCl2 HClD.H2SO4 MgCl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨһƿ���õ��ռ����������Ƶĺ�����ijͬѧȡ25.0g��Ʒ������ˮ�õ�100g��Һ���ټ���100g����ϡ���ᣬ��ַ�Ӧ�����ٲ������壬�����Һ����Ϊ197.8g������㣺
��1����Ӧ����CO2��������
��2����Ʒ��NaOH���������������������0.1%����
��3�����������Һ�е���Ԫ�ص����������������0.1����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���밸��
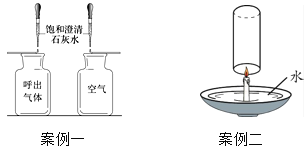
��1������һ̽��������������ж�����̼�����ȿ����еĸߣ�ʵ����������Ҫ��������ʢװ������Ʒ�ļ���ƿ��С�����ͬ�⣬��Ҫ����_____��ͬ��
��2���������ò�����Ѹ�ٿ�סȼ�յ�����ʹ����ʼ�ս�û��ˮ�С�������ʵ��ʵ������_____
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Դ����Լ��Դ���������������Ǵ�ҹ�ͬ��ע��������⡣
��1��������ȼ����________
��2���ҹ����䡰�϶�һ�š��Ļ��ʹ��C2H8N2��ƫ�����£���N2O4Ϊ�ƽ���������ɸû�ѧ����ʽ��C2H8N2+2N2O4��2CO2+3_____+4H2O
��3��SO2�Ĵ����ŷ����γ����꣬pHС��_____�Ľ����Ϊ���꣬ij��糧��ȼú�м���ʯ��ʯ�Խ��ͷ�����SO2�ĺ���������ȼ�պ�õ�50t���������÷�Һ�к���Ԫ�ص���������Ϊ24%���൱�����ŷ�_____t��������
��4����Ȼ��ȼ�ղ����ʱ���ɵ�������_____�����������ȼ������������
��5������þ�Ż���ʹ�ö�����̼������Ϊþ���ڶ�����̼�м���ȼ�գ�����һ�ֺ�ɫ���ʺ�����þ���ֹ����ĩ��д���÷�Ӧ�Ļ�ѧ����ʽ________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ��װ�ã��ش��й����⡣
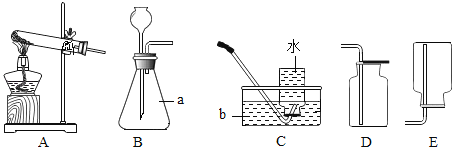
��1��д������a��b�����ƣ�a_____��b_____��
��2��ʵ������Aװ�ò���������������ѧ��Ӧ�Ļ�ѧ����ʽΪ_____�����Ҫ�ռ��ϴ���������ѡ����ռ�װ��Ϊ_____��
��3��ʵ������ʯ��ʯ��ϡ���ᷴӦ��ȡ���ռ�������̼��Ӧѡ���װ��Ϊ_____������ĸ�����䷴Ӧԭ��Ϊ_____��
��4��þ���Ͻ�M�˺����������ᷴӦ����H2 0.1�ˣ���M������_____��
A 0.8 B 1 C 1.5 D ��ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����к�HCl��CuCl2�Ļ����Һ50g�������Һ����μ���������������Ϊ10%��NaOH��Һ�����ɳ��������������NaOH��Һ��������ϵ��ͼ��ʾ��
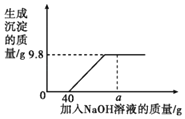
��1��ǡ����ȫ��Ӧʱ������NaOH��Һ��������Ϊ_____g��
��2����ǡ����ȫ��Ӧʱ������Һ���ʵ���������_____���������1%����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com