
����ʹ�õ�ˮ���ײ�������һ��ˮ��������Ҫ�ɷ���̼��ƺ�������þ��Ϊ��ȷ�ⶨˮ����������þ�ĺ�����ʵ��С��ֱ�ȡ����ͬ����ˮ����Ʒ��7.00g��������ͼ��ʾװ��������������ʵ�飬����ÿ��ʵ����װ��B�������仯��¼���±���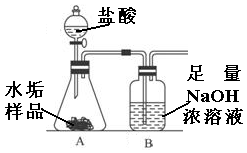
| | ��һ�� | �ڶ��� | ������ | ƽ��ֵ |
| Bװ�����ӵ�������g�� | 2.17 | 2.22 | 2.21 | |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
ijͬѧ��ʵ������ʧȥ��ǩ��ʯ��ʯ��Ʒ���д��ȣ���Ʒ��̼��Ƶ������������ⶨ��ȡ����ʯ��ʯ��Ʒ12.5g������94.4gһ������������ϡ���ᣮ��ַ�Ӧ���ռ���4.4g������̼���壨ʯ��ʯ�е����ʼȲ�����ˮ��Ҳ����ϡ���ᷴӦ������
��1��ʯ��ʯ��Ʒ��̼��Ƶ�����������
��2����Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
100gijһ������������ϡ����ǡ����13gп��ȫ��Ӧ������㣺
��1����Ӧ���������������� ��g��
��2��ϡ���������ʵ�������������д��������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��10.6g̼������50gϡ�������ͬһ�ձ��У�����ǡ����ȫ��Ӧ���Լ��㣺ϡ���������ʵ�������������д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
������������Һ�ⶨij������Һ���ʵ�����������ʵ�����£�ȡ25g��������Һ�����뵽�ձ��У�Ȼ���50g��������Ϊ10%������������Һ���ϵ����ձ��У�ͬʱ�õ���PH�Ʋ��϶�ȡ��Ӧʱ��PH���ó���ͼ��ʾ�Ĺ�ϵ����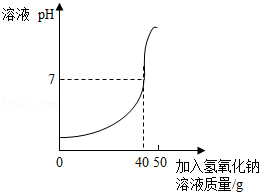
��1����������Һ���ʵ�����������
��2������500g����������Һ��������������Ϊ98%��������Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��1����������SO3���У���Ԫ������Ԫ�ص����������� ������Ԫ�ص������������� ����
��2������ij�ȼ���ŷŵ�β���ﺬ�ж���������Cl2����Ϊ��ֹ����Ⱦ������������20%��NaOH��Һ������������Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH=NaClO+NaCl+H2O�������㣺4t������������Ϊ20%��NaOH��Һ�������Ͽ���������������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
С��ͬѧ���ڿ����з���һ��ʱ��ġ�ͭ��������˿����������ͭ��Һ�Ƴ�����ͼ���ĵijɷ�ʱ�з�����ȡ��64.1�˹�����Ʒ����10%���������ܽ⣬��Һ����ɫ�������������ʣ�����������10%�����������仯��ϵ��������ͼ��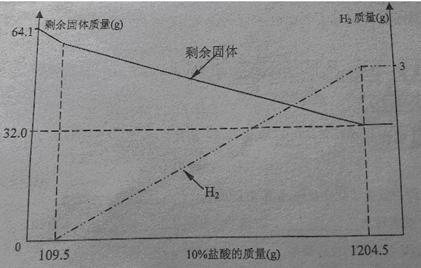
���ͼ�����ݷ�����
��1����ͼ��֪��CuԪ�ص�����_____g��64.1g��ͭ������Cu��Al��_____���ѧʽ��
��2�����ϻ�ѧ����ʽ����á�ͭ������AlԪ�ص�����������
��3��ֻ֪����ͭ��������m1������10%��������������m2��,Ҳ�������ͭ��������Ԫ�����������������ʽΪ_______________(��m1��m2��ʾ���ɲ�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
һ������������β���е�һ�ִ�����Ⱦ�����һ����ɫ���壬������ˮ���ܶȱȿ����Դ��ڿ�������������Ѹ�ٷ�Ӧ���ɺ���ɫ�Ķ����������塣��ʵ�������ռ�һ���������Բ��õķ����ǣ��� ��
| A����ˮ������ | B�������ſ��������� |
| C�������ſ��������� | D�������ſ�������������ˮ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��ͼ��ijͬѧ��Ƶ�ʵ��װ�ã�����ʵ������ȡ�����ʵ��̽��������˵����ȷ���ǣ�������
| A������a�dz���©�� |
| B������b��ʵ���п�ֱ�Ӽ��� |
| C����װ�ÿ����ڸ��������ȡ���� |
| D����װ�ÿ���ȡ���ռ������������̼ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com