
����Ŀ����������̼��Ƶijɷ���CaCO3������ζ�İ�ɫ��ĩ����Ӧ����ͭ��ֽ����Ʊֽ��ֽ��Ʒ�У��������ֽ��Ʒ���ȶ��ԡ�Ӳ�Ⱥ������ԡ����ڹ��ڵĹ�ҵ����������Ҫ��̼�����������������£�
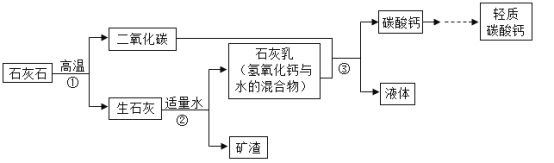
��1�����������IJ���������_________________________________��
��2�����������У��������Ϸ�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��3��ʯ��ʯ������̼��ƵIJ���ǣ�д��һ����_________________________________ ��
�����������������Ľ�������������Դ���ۺ������Ǵ�����������¯ұ��������������ͼ��ʾ��������������ͼ�ش����⡣
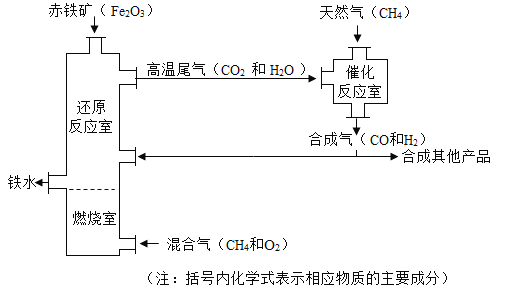
��1���ù��յ���Ҫԭ��Ϊ������_________________________________��
��2����������CO��Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��3���ڴ���Ӧ���У����ϼ۷����ı��Ԫ���ǡ�__________________________
���𰸡����� CaO+H2O=Ca(OH)2 ʯ��ʯ�ǻ�������̼��Ƹ����������Ȳ�ͬ����ʯ��ʯ�ǿ�״���壬����̼����Ƿ�ĩ����������С��ͬ�� ��������Ȼ�� 3CO +Fe2O3![]() 2Fe +3CO2 C��H��̼Ԫ�غ���Ԫ�أ�
2Fe +3CO2 C��H��̼Ԫ�غ���Ԫ�أ�
��������
����1��������ǰѲ�����ˮ��̼�����Һ�������ǹ��ˣ�
��2����������ˮ��Ӧ�������������ǻ��Ϸ�Ӧ��
��3��ʯ��ʯ�ǻ�������̼��Ƹ�����
�𰸣���1�����ˣ���2��CaO+H2O�TCa��OH��2����3��ʯ��ʯ�ǻ�������̼��Ƹ������Ȳ�ͬ����
����1����ͼ��֪���ù��յ���Ҫԭ��Ϊ������������ʯ����Ȼ���ȣ������������Ȼ����
��2��һ����̼�����������·�Ӧ�������Ͷ�����̼�����3CO+Fe2O3![]() 2Fe+3CO2��
2Fe+3CO2��
��3��������̼�У�̼Ԫ����+4�ۣ���һ����̼�У���+2�ۣ���ˮ�����У�����+1�ۣ������У�����0�ۣ����C��H��̼Ԫ�غ���Ԫ�أ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ�����ǵĺ�ˮ�����ձ��У��ȼ��������������ܽ⡢����һ���������ͼװ�ý��й��ˡ���ش�:
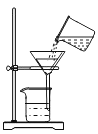
(1)���õ���������_______��
(2)ͼ�л�ȱ��һ��������_______����������______��©���¶˽����ձ��ڱڵ�Ŀ����_______��
(3)���������У����ֹ����ٶ�̫����ԭ����_______��
(4)���˺õ��˳�������ˮ�����˷ܵ�����:���������Ƶ��˴�ˮ!������˵������?________��������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��Ӧ���۹�����ͼ��������˵���У�����������
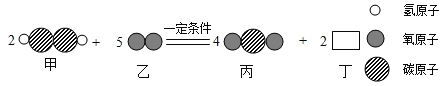
A. �÷�Ӧ����������Ӧ
B. �ס��������ʲμӷ�Ӧ��������Ϊ13��16
C. ���ʶ��Ļ�ѧʽ��H2O
D. ��Ӧǰ����Ԫ�صĻ��ϼ۷����˸ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ͬѧ���������ᴿʵ��IJ���ʾ��ͼ��
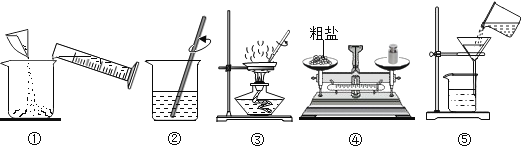
��ش���������:
��1�������ᴿʵ��IJ���˳��Ϊ���������ţ�_____��
��2��ʵ������ж���õ��˲�����,�����ø��в�ͬ,д������һ�ֲ�����������_____����ѡһ��д����
��3���ڽ��в�����ʱ,����������_____ʱֹͣ����;�����������еĴ���_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���û�ѧ���ű�ʾ��д����������ʾ�ĺ��壺
��2����ԭ��___________��
��2H2____________��
��2��������__________��
��+4���̵�������__________��
��̼�����__________��
������������Ԫ�ػ��ϼ�Ϊ+3_________��
���Ȼ����е�������_____ ��
�ౣ��ˮ���ӻ�ѧ���ʵ���С����____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д�����з�Ӧ�Ļ�ѧ����ʽ��
��1����̼��������������ͭ��ĩ��Ϲ��ȣ�_____��
��2������Ƭ��������ͭ��Һ�У�_____��
��3���ó����ʯ��ˮ���������̼��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������1000t��Fe2O380%�ij�����ʯ�������в�����Ԫ�أ����Լ��㣺
��1���ó�����ʯ��Fe2O3��������_____t��
��2���ó�����ʯ����Ԫ�ص�������_____t��
��3���������ܵõ�������8%��������������_____t����������ȷ��0.1t����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�ⶨij�ֱ�����̼��Ƶĺ�����ȡ25g���ǣ�����ϡ���ᣬ��ַ�Ӧ���ʣ��������������ϡ�����������ϵ��ͼ��ʾ(��֪���ʲ����뷴Ӧ��Ҳ������ˮ)����㣺
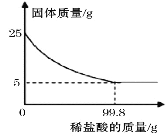
��1�����ֱ�����̼��Ƶ����������Ƕ��٣�__________ (̼��Ƶ���������=![]() )��
)��
��2��25g������ϡ����ǡ����ȫ��Ӧʱ�����ɶ�����̼����������?__________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ������������������ϵ���С�
��1������ij��Ȼˮ��Ӳˮ������ˮ��ȡ�����������ˮ�����裬��ĭ�����а�ɫ��״�����Ȼˮ��_________��
��2����ͥ��Ӳˮת��Ϊ��ˮ�ķ���__________��
��3����ͼ1�ǵ��ˮ�ļ���װ��ͼ��
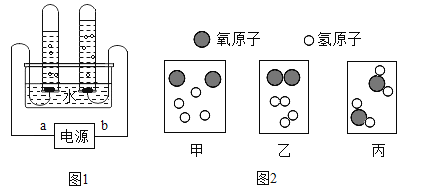
��ͼ1��Դ����a�ǵ�Դ��______��������������b�������Թ��������������______�����������ķ�����________
��4��������ͼ2����ʾˮ�ֽ�Ϊ���������������г��ֵ���ʾ��ͼ���밴�����ڻ�ѧ�仯�����г��ֵ�˳������______���üס��ҡ�����ʾ����
��5�����ˮ��Ӧ��ѧ����ʽΪ ___________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com