
| ������Ŀ | ʵ��ǰ | ʵ��� | |
| �ձ���ˮ����� | �ձ���ʣ��ˮ����� | ����ƿ���۳�������͵��ܵ��ݻ� | |
| ���/mL | 80.0 | 54.5 | 126.0 |
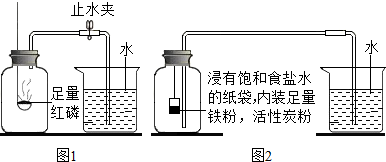
���� �������еĿ��������������IJⶨԭ�����з��������ȼ���������������ף����屻���ģ�ѹǿ��С���ʻ����ˮ�������������������֪ʶ���з�����ɣ�
��� �⣺��1����ȼ���������������ף��÷�Ӧ�Ļ�ѧ����ʽΪ��4P+5O2 $\frac{\underline{\;��ȼ\;}}{\;}$2P2O5��
��2������ȼ������������ʹƿ�ڵ���ѹ��С��ˮ������
��ʵ��Ľ�����1������������ˮ��Ӧ��������������[Fe��OH��2]���÷�Ӧ�Ļ�ѧ����ʽΪ��2Fe+2H2O+O2�T2Fe��OH��2��
��2�����ݷ�Ӧǰ���ձ���ˮ������仯���Կ��������ĵ�����������ǣ�80-54.5=25.5mL�����������������Ϊ��$\frac{25.5mL}{126mL}$��100%=20.2%��
��3��ʹ�����Ļ�����������������ʹ�������ĵĸ�Ϊ���ף�ʵ������ȷ������ʱ���ǵ����ݻ��Ϳ۳��������ļ���ƿ�������ʹʵ������Ϊȷ��
�ʴ�Ϊ����ʵ��عˡ���1��4P+5O2 $\frac{\underline{\;��ȼ\;}}{\;}$ 2P2O5��
��2����ѹ��
��ʵ��Ľ�����.2Fe+2H2O+O2=2Fe��OH��2��
��1��20.2%��
��2�������Ļ�������ʹ����ƿ�е��������ĸ����ף�ʹʵ������ȷ��
�ڲ���ʱ���ǵ������ݻ��Ϳ۳��������ļ���ƿ�ݻ���ʹʵ������ȷ��
���� ���⿼����������ĺ����IJⶨ�Լ�ʵ��װ�õĸĽ�����ɴ��⣬�����������е�֪ʶ���У�


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 2013�� 6����Ѯ������ʮ�ŷɴ�������3������Ա�������գ��롰�칬һ�š�Ŀ�����������Խӣ������칬һ��Ŀ��������������ص��ܷ�ӦʽH2+2NiO��OH���T2Ni��OH��2��������������ȷ���ǣ�������
2013�� 6����Ѯ������ʮ�ŷɴ�������3������Ա�������գ��롰�칬һ�š�Ŀ�����������Խӣ������칬һ��Ŀ��������������ص��ܷ�ӦʽH2+2NiO��OH���T2Ni��OH��2��������������ȷ���ǣ�������| A�� | H2���ڵ��� | |
| B�� | NiO��OH�����⡢��Ԫ�ص�������Ϊ1��2 | |
| C�� | Ni��OH��2��Ni���ϼ�Ϊ+2�� | |
| D�� | �μӷ�Ӧ��H2��NiO��OH����������Ϊ1��92 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | +5 | B�� | +1 | C�� | +2 | D�� | -1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ˫��ˮ������ | ˫��ˮ��Ũ�� | MnO2������ | ��ͬʱ���ڲ���O2����� | |
| I | 50.0g | 1% | 0.1g | 9mL |
| II | 50.0g | 2% | 0.1g | 16mL |
| III | 50.0g | 4% | 0.1g | 31mL |
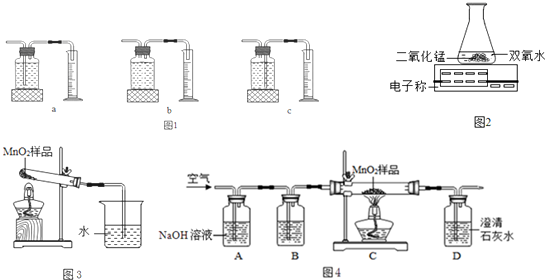
| ʵ�鲽�輰���� | ���� |
| ��ͼ3װ�ã����ȿյ��Թܣ���һ�˵ij���ʯ��ˮû�б���� | ���費������������������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
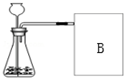 �о���ѧϰС��ѡ��H2O2 ��Һ�ֽ�����O2�ķ�Ӧ������ʲô�����йء��Ŀ������̽��������������̽������Ҫ���̣�
�о���ѧϰС��ѡ��H2O2 ��Һ�ֽ�����O2�ķ�Ӧ������ʲô�����йء��Ŀ������̽��������������̽������Ҫ���̣�| ʵ���� | 1 | 2 |
| ��Ӧ�� | 5%H2O2��Һ | 5%H2O2��Һ |
| ���� | 1gˮ��� | 1gMnO2 |
| ʱ�� | 165�� | 46�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
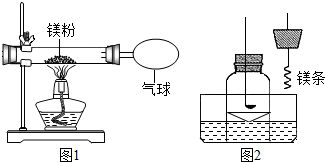 ij��ȤС��Ϊ��֤�����غ㶨�ɣ�����þ���ڿ�����ȼ�յ�ʵ�飮
ij��ȤС��Ϊ��֤�����غ㶨�ɣ�����þ���ڿ�����ȼ�յ�ʵ�飮| ʵ����� | ʵ�������� |
| ȡ������ɫ�������Թ��У�����������ˮ������ʪ��ĺ�ɫʯ����ֽ�����Թܿ� | �Թ��������������ʪ��ĺ�ɫʯ����ֽ������֤��������ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com