


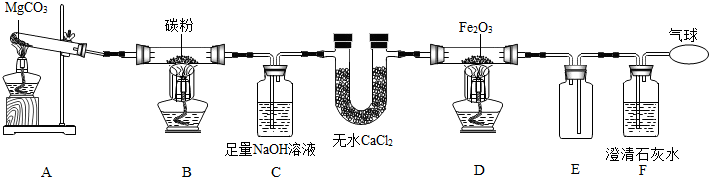
���� ��1������̼������Ǽ��ٶ�����̼���ŷ�����
��2������Ԫ�����ڱ��е�һ��С���е���Ϣ��֪Ԫ�ص����ơ�Ԫ�ط��š������������ԭ������������ɣ�
��3������Ŀ��Ϣ��֪�������к���̼Ԫ�أ����������������˱�����ʯ�ң�����̼��ƣ����Ƕ�����̼��������ʯ�ң�
��4�����Ը�������֮������õ�����ж�ʵ��������Ҫ������ѹǿ�ı仯����������⣬��װ���е�ѹǿ���������U�ι��е�Һ������Ҹߣ����������غ㶨����ȷ����д��ѧ����ʽ��
��5�����������غ㶨�ɣ���Ӧǰ����ٵ�����Ϊ������̼������������̼��������ᷴӦ�Ļ�ѧ����ʽ�����ݶ�����̼�����������̼��Ƶ�����������������Ʒ�����ʵ��������������ʯ��ʯ�����ʵ�����������
��� �⣺��1������̼������Ǽ��ٶ�����̼���ŷ�����
�ʴ�Ϊ��CO2��
��2��A����̼��ƫ��Ϊ��ʯ������̼Ԫ��Ϊ�ǽ���Ԫ�أ���A˵����ȷ��
B������Ϣ��֪��̼ԭ�ӵ�������Ϊ6����B˵����ȷ��
C��̼ԭ�ӵ�������Ϊ6����ԭ�ӽṹ������������ӦΪ4����C����
D������Ϣ��֪��̼ԭ�ӵ����ԭ������Ϊ12.01����D˵����ȷ��
��ѡC��
��3�������к���̼Ԫ�أ�����������������Կ���������ʯ�ң�����̼��ƣ����Ƕ�����̼����˼Ļ�ѧʽΪCaCO3����ת��Ϊ���Ļ�ѧ����ʽΪCaO+H2O=Ca��OH��2��
��4���������֪����֮�������ʱӦ����ʹ��ƿ�е�ѹǿ����ѹǿ�����ԭ�������ֿ��ܣ�һ����ƿ�������ʷ�Ӧ�������壬��ʹƿ������ѹǿ�����ý������ᷴӦ��̼���������ᷴӦ��˫��ˮ�ڶ������̴������²������������ԣ���һ�����������������ܽ�ų�������ʹƿ�ڿ����������ͣ�ѹǿ������Ũ��������ˮ������������ˮ����ѧ����ʽΪ��CaO+H2O=Ca��OH��2�� ˫��ˮ�ڶ������̴�����������ˮ����������Ӧ�Ļ�ѧ����ʽΪ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��5�����������غ㶨�ɿ�֪����CO2������Ϊ��12.5g+50 g-58.1g=4.4g
��ʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl�TCaCl2+CO2��+H2O
100 44
x 4.4g
$\frac{100}{x}=\frac{44}{4.4g}$
x=10g
������Ʒ�����ʵ�������12.5g-10g=2.5g
ʯ��ʯ�����ʵ���������Ϊ��$\frac{2.5g}{12.5g}$��100%=20%
�ʴ�Ϊ��
��1��CO2��
��2��C
��3��CaCO3��CaO+H2O=Ca��OH��2��
��4��CaO+H2O=Ca��OH��2��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��5��ʯ��ʯ�����ʵ���������Ϊ20%��
���� ���⿼��ѧ������Ԫ�����ڱ��е�һ��С��������ȡ��Ϣ���ϰ�⣬����ѧ��������Ϣ������Ϣ������������ȷ��ͬλ�õ����ֵ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
 ������������ʵ���У����dz��ᷢ�����������������һЩ���ݲ�������������ô�����ģ������һ���⣬ͬѧ�ǿ�չ����̽����
������������ʵ���У����dz��ᷢ�����������������һЩ���ݲ�������������ô�����ģ������һ���⣬ͬѧ�ǿ�չ����̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ռNa2CO3���� | B�� | ��ʯ�ҡ�CaCO3���� | ||
| C�� | ��ʯ�ҡ�CaO�������� | D�� | ̼����李�NH4HCO3���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʱ������ȼ���̻����� | |
| B�� | ������������Ϯʱ���������ٻ��� | |
| C�� | ��������������ֱ�������0.1��1.0�ף����Թ�������Ҫ���� | |
| D�� | Ϊ�˼�СPM2.5�Ի�����Ӱ�죬��ֹ��ͥʹ��˽�ҳ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ��̽����

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com