
ЁОЬтФПЁПЛЏбЇПЮКѓЃЌЛЏбЇаЫШЄаЁзщЕФЭЌбЇдкећРэЪЕбщзРЪБЃЌЗЂЯжгавЛЦПЧтбѕЛЏФЦШмвКУЛгаШћЯ№ЦЄШћЃЌеїЕУРЯЪІЭЌвтКѓЃЌПЊеЙСЫвдЯТЬНОПЃК
[ЬсГіЮЪЬт1] ИУЧтбѕЛЏФЦШмвКЪЧЗёБфжЪСЫФиЃП
[ЪЕбщЬНОП1]
ЪЕбщВйзї | ЪЕбщЯжЯѓ | ЪЕбщНсТл |
ШЁЩйСПИУШмвКгкЪдЙмжаЃЌЯђШмвКжаЕЮМгЯЁбЮЫсЃЌВЂВЛЖЯеёЕДЁЃ | _____________ | ЧтбѕЛЏФЦШмвКвЛЖЈБфжЪСЫЁЃ |
[ЬсГіЮЪЬт2] ИУЧтбѕЛЏФЦШмвКЪЧШЋВПБфжЪЛЙЪЧВПЗжБфжЪФиЃП
[ВТЯыгыМйЩш]
ВТЯы1ЃКЧтбѕЛЏФЦШмвКВПЗжБфжЪЁЃ ВТЯы2ЃКЧтбѕЛЏФЦШмвКШЋВПБфжЪЁЃ
[ВщдФзЪСЯ] ЂХ ТШЛЏИЦШмвКГЪжаадЁЃ
ЂЦ ТШЛЏИЦШмвКФмгыЬМЫсФЦШмвКЗДгІ(ЗНГЬЪН)ЃК________________________ЁЃ
[ЪЕбщЬНОП2]
ЪЕбщВНжш | ЪЕбщЯжЯѓ | ЪЕбщНсТл |
ЂХШЁЩйСПИУШмвКгкЪдЙмжаЃЌЯђШмвКжаЕЮМгЙ§СПЕФТШЛЏИЦШмвКЃЌВЂВЛЖЯеёЕДЁЃ | га________ЩњГЩ | ЫЕУїдШмвКжавЛЖЈгаЬМЫсФЦЁЃ |
ЂЦШЁВНжшЂХЪдЙмжаЕФЩйСПЩЯВуЧхвКЃЌЕЮМгЗгЬЊШмвКЁЃ | _____________ | ЫЕУїдШмвКжавЛЖЈга______ЁЃ |
[ЪЕбщНсТл] ИУЧтбѕЛЏФЦШмвК_______(ЬюЁАВПЗжЁБЛђЁАШЋВПЁБ)БфжЪЁЃ
[ЗДЫМгыЦРМл] ЂХЧтбѕЛЏФЦШмвКТЖжУгкПеЦјжаШнвзБфжЪЃЌЧыаДГіЯрЙиЗДгІЕФЛЏбЇЗНГЬЪНЃК_________ЁЃ
ЂЦдкЩЯЪі[ЪЕбщЬНОП2]жаЃЌаЁУїЬсГіПЩгУЧтбѕЛЏИЦШмвКДњЬцТШЛЏИЦШмвКЃЌФуШЯЮЊИУЗНАИ________(ЬюЁАПЩааЁБЛђЁАВЛПЩааЁБ)ЁЃ
[РэНтгыгІгУ] ЧтбѕЛЏФЦШмвКШнвзБфжЪЃЌБиаыУмЗтБЃДцЁЃ
ЁОД№АИЁП гаЦјХнУАГі Na2CO3+CaCl2= CaCO3![]() + 2NaCl АзЩЋГСЕэЩњГЩЃЛ БфКь ЧтбѕЛЏФЦЃЛ ВПЗжБфжЪ 2NaOH+CO2===Na2CO3+H2O ВЛПЩаа
+ 2NaCl АзЩЋГСЕэЩњГЩЃЛ БфКь ЧтбѕЛЏФЦЃЛ ВПЗжБфжЪ 2NaOH+CO2===Na2CO3+H2O ВЛПЩаа
ЁОНтЮіЁПЧтбѕЛЏФЦБфжЪКѓЩњГЩЬМЫсФЦЃЌЙЪМгШыЯЁбЮЫсКѓЃЌвЛЖЈгаЦјХнУАГіЃЛ
ТШЛЏИЦгыЬМЫсФЦЗДгІЩњГЩЬМЫсИЦКЭТШЛЏФЦЃЌЗДгІЗНГЬЪНЮЊNa2CO3+CaCl2= CaCO3![]() + 2NaCl
+ 2NaCl
дШмвКжагаЬМЫсФЦЃЌдђМгШыТШЛЏИЦКѓгаАзЩЋГСЕэЩњГЩЃЛЯђЩЯЧхвКжаМгШыЗгЬЊЃЌЗњБфЮЊКьЩЋЃЌдђЫЕУївЛЖЈгаЧтбѕЛЏФЦЃЛдђЫЕУїИУШмвКВПЗжБфжЪЃЛЧтбѕЛЏФЦгыПеЦјжаЕФЖўбѕЛЏЬМЗДгІЩњГЩЬМЫсФЦКЭЫЎЃЌЙЪБфжЪЕФЗДгІЗНГЬЪНЮЊ2NaOH+CO2===Na2CO3+H2O
ШчЙћгУЧтбѕЛЏИЦШмвКДњЬцТШЛЏИЦШмвКЃЌЧтбѕЛЏИЦБОЩэЯдМюадЃЌЙЪЛсгАЯьЧтбѕЛЏФЦЕФХаЖЯЃЌЙЪВЛФмгУЧтбѕЛЏИЦДњЬцТШЛЏИЦЃЛ


 аЧМЖПкЫуЬьЬьСЗЯЕСаД№АИ
аЧМЖПкЫуЬьЬьСЗЯЕСаД№АИ УЂЙћНЬИЈДяБъВтЪдОэЯЕСаД№АИ
УЂЙћНЬИЈДяБъВтЪдОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪвРяЃЌдквЛИіОпгаПЬЖШКЭПЩвдЛЌЖЏЕФЛюШћЕФВЃСЇЙмжаЗХШыАзСз(Й§СПЃЌАзСзШМЩеЫљашЕФзюЕЭЮТЖШЮЊ40 ЁцЃЌШМЩеЯжЯѓМАВњЮяОљгыКьСзЯрЭЌ )ЃЌНЋВЃСЇЙмЙЬЖЈКУЃЌЗХдкЪЂга80 ЁцШШЫЎЕФЩеБЩЯЃЌЪЕбщзАжУШчЭМ1ЫљЪОЃЌЪдЛиД№ЯТСаЮЪЬтЁЃ
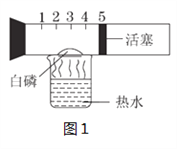

ЃЈ1ЃЉећИіЪЕбщЙ§ГЬжаЃЌПЩвдЙлВьЕНЕФЯжЯѓгаЃК
ЂйВЃСЇЙмФк______________________ЃЛ
ЂкЛюШћ________________________________________________ЃЛ
ЂлЛюШћзюКѓдМЭЃдкПЬЖШ_______ДІЁЃ
АзСздкЗДгІЙ§ГЬжавЊЙ§СПЕФдвђЪЧ_________________________________ЁЃ
ЃЈ2ЃЉЭМ2ЮЊЪщБОЩЯВтЖЈПеЦјжабѕЦјКЌСПЕФЪЕбщзАжУЃЌЭМ1КЭЭМ2ЯрБШЃЌФуШЯЮЊФФжжзАжУИќКУЃПРэгЩЪЧЪВУДЃП___________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМЪЧАВзАдкЦћГЕХХЦјЯЕЭГжазюживЊЕФЛњЭтОЛЛЏзАжУЗЂЩњЕФЛЏбЇЗДгІЕФЮЂ
ЙлЪОвтЭМЃЈ4CO + 2NO2![]() 4CO2 + N2 ЃЉЁЃ
4CO2 + N2 ЃЉЁЃ
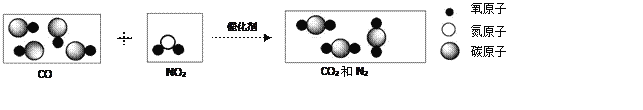
ЃЈ1ЃЉЧыдкЬтЭМЕкЖўЁЂЕкШ§ЗНПђжаВЙШЋЮЂЙлСЃзгЁЃ___________ЁЂ___________
ЃЈ2ЃЉCOЛЙдбѕЛЏЭЕФЪЕбщЯжЯѓЪЧ___________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЪЧЧтбѕЛЏФЦШмвКгыЯЁСђЫсШмвКЧЁКУЭъШЋЗДгІЕФЮЂЙлЪОвтЭМЃЌгЩДЫЕУГіЕФНсТлВЛе§ШЗЕФЪЧ( )
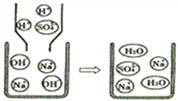
A. ЗДгІНсЪјЪБШмвКЕФpH=7
B. ЗДгІЧАКѓУЛгаЗЂЩњБфЛЏЕФЮЂСЃЪЧNa+КЭSO42-
C. ИУЗДгІЪЧNa+КЭSO42-НсКЯЩњГЩNa2SO4
D. ИУЗДгІЕФЪЕжЪЪЧH+КЭOH-НсКЯЩњГЩH2OЗжзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПO2ЁЂH2ЁЂCO2ЪЧжабЇЛЏбЇжаГЃМћЕФЦјЬхЃЌЧыИљОнЯТСазАжУЭМЛиД№ЮЪЬтЃК
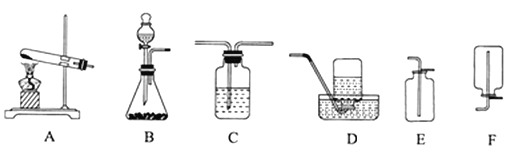
ЃЈ1ЃЉЪЕбщЪвжЦШЁCO2ЕФЗЂЩњзАжУгІбЁгУ_____ЃЈгУзжФИAЁЋFЬюПеЃЉзАжУЃЌЦфжаЕФВЃСЇвЧЦїЃЈСЌНгзАжУГ§ЭтЃЉЕФУћГЦЪЧ________ЃЛ
ЃЈ2ЃЉЪЕбщЪвгУBзАжУжЦШЁO2ЪБЃЌМгШыЕФЙЬЬхЦ№______зїгУЃЛИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______ЁЃ
ЃЈ3ЃЉЪЕбщЪвгУCзАжУИЩдяH2ЪБМгШыЕФЪдМСЪЧ_____ЃЈЬюУћГЦЃЉЃЌЪеМЏH2бЁгУЕФзАжУЪЧDЛђFЃЌЦфдвђЪЧ________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПРЯЪІЮЊЭЌбЇУЧЬсЙЉСЫШчЭМЫљЪОЕФЪЕбщзАжУЃК
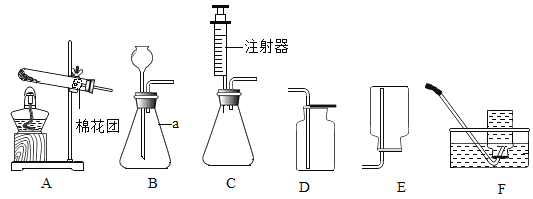
(1)аДГіaжавЧЦїУћГЦЃК _______________
(2)ШєвЊЪеМЏвЛЦПбѕЦјЃЌЙЉЁАЬњдкбѕЦјжаШМЩеЁБЪЕбщЪЙгУЃЌзюКУбЁдёЕФЦјЬхЪеМЏзАжУЪЧFЃЌРэгЩЪЧЂй_______ЃЌЂк ___________________ ЃЎ
(3)ЪЕбщЪвжЦШЁЖўбѕЛЏЬМЗЂЩњзАжУПЩбЁгУзАжУBЛђзАжУCЃЌзАжУCгызАжУBЯрБШЃЌзАжУCгХЕуЪЧ______ЃЎЪЕбщЪвжЦШЁЖўбѕЛЏЬМЕФЛЏбЇЗНГЬЪНЮЊ _______________ ЃЎ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГаЫШЄаЁзщЭЌбЇШЅбЮГЁВЮЙлЃЌДјЛиСЫВПЗжДжбЮбљЦЗЃЌВЂЖдЦфНјааСЫШчЯТЬНОПЃК
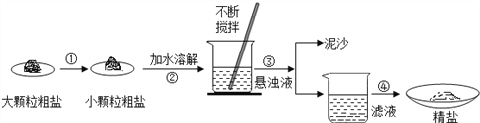
(1)ВйзїЂлЕФУћГЦЪЧ_________________
(2)ВйзїЂмжагУЕНВЃСЇАєЃЌзїгУЮЊ_____________________ЁЃ
(3)ЪЕбщНсЪјКѓГЦСПЛёЕУЕФОЋбЮЃЌВЂМЦЫуОЋбЮЕФжЦЕУТЪЃЌЗЂЯжжЦЕУТЪНЯЕЭЃЌЦфПЩФмдвђЪЧ__________(ЬюађКХ)ЁЃ
AЃЎЪГбЮУЛгаШЋВПШмНтМДЙ§ТЫ BЃЎеєЗЂЪБЪГбЮЗЩНІОчСв
CЃЎеєЗЂКѓЃЌЫљЕУОЋбЮКмГБЪЊ DЃЎЦїУѓЩЯеДгаЕФОЋбЮУЛШЋВПзЊвЦЕНГЦСПжНЩЯЁЃ
(4)ВщдФзЪСЯЕУжЊЃКДжбЮжаГ§КЌЩйСПФрЩГЕШВЛШмаддгжЪЭтЃЌЛЙКЌгаЩйСПЕФПЩШмаддгжЪ(МйЖЈПЩШмаддгжЪжЛгаMgCl2вЛжж)ЃЌЮЊСЫЕУЕННЯДПОЛЕФТШЛЏФЦЃЌаЁзщЭЌбЇНЋЫљЕУЕФЁАОЋбЮЁБгжзїСЫШчЯТДІРэЃК
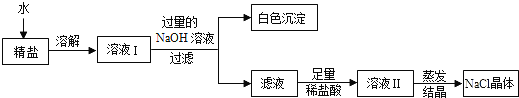
ЂйАзЩЋГСЕэЕФЛЏбЇЪНЮЊ_______ЁЃ
ЂкдкЕУЕНЕФТЫвКжаМгШызуСПЕФЯЁбЮЫсЕФФПЕФЪЧ____________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђ10.0gФГЪЏЛвЪЏ(дгжЪВЛШмгкЫЎЧвВЛВЮМгЗДгІ)жаЯШКѓЕЮМг100.0gЯЁHClКЭвЛЖЈжЪСПNa2CO3ШмвКЃЌЗДгІЙ§ГЬжаМгШыШмвКЕФжЪСПгыВЛШмЙЬЬхЛђВњЩњЦјЬхЕФжЪСПЙиЯЕШчгвЭМЫљЪОЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ
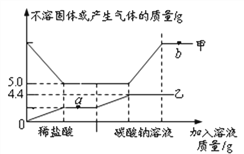
A. МзБэЪОВЛШмЙЬЬхЕФжЪСП
B. aЕуЖдгІШмвКжаЕФШмжЪжЛга1жж
C. ИУЯЁбЮЫсЕФШмжЪжЪСПЗжЪ§ЮЊ7.3%
D. МьбщbЕуЖдгІШмвКжаЕФШмжЪЃЌПЩЯШЕЮМгзуСПЕФAgNO3ШмвКЃЌОВжУКѓдйЕЮМгЩйСПCa(NO3)2ШмвК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКГѕжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬМЫсвћСЯЪЧжИдквЛЖЈЬѕМўЯТГфШыЖўбѕЛЏЬМЦјЬхЕФвћСЯЃЌШчПЩРжЁЂбЉБЬЁЂЦћЫЎЕШЁЃЩњВњЬМЫсвћСЯЕФжївЊСїГЬШчЯТЃК
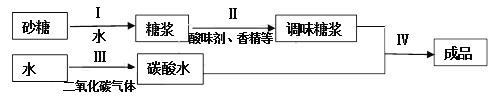
(1)ВНжшЂёЕФВйзїЪЧ_________ЁЃ
(2)ВНжшЂѓжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_________ЃЌЦфЗДгІРраЭЮЊ_________ЁЃ
(3)ГЩЦЗвћСЯжаЫљКЌЕФШмжЪга_________(жСЩйаДГі2жж)ЃЌаДГіГЩЦЗвћСЯжаКЌгаЕФЮЂЙлСЃзгЗћКХ_________(жСЩйаДГі2жж)ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com