
ΓΨΧβΡΩΓΩΧΦΚΆΧΦΒΡΜ·ΚœΈο‘ΎΉ‘»ΜΫγ÷–ΙψΖΚ¥φ‘ΎΘ°«κΜΊ¥πΘΚ
Θ®1Θ©ΫπΗ’ ·ΚΆ ·ΡΪ «ΝΫ÷÷≥ΘΦϊΒΡΧΦΒΞ÷ Θ§ΤδΈοάμ–‘÷ œύ≤νΚή¥σΒΡ‘≠“ρ «________Θ°
Θ®2Θ©œ¬Ν– «”–ΙΊCΓΔCOΓΔCO2»ΐ÷÷Έο÷ ΒΡΥΒΖ®ΘΚ
ΔΌ»ΐ÷÷Έο÷ ΕΦΚ§”–ΧΦ‘ΣΥΊΘ§ΕΦΩ…“‘»Φ…’
ΔΎCOΓΔCO2ΕΦ «ΟΜ”–―’…ΪΓΔΟΜ”–ΤχΈΕΒΡΤχΧε
ΔέCO2Ω…”Ο”ΎΙβΚœΉς”ΟΘ§COΩ…”Ο”Ύ»ΥΙΛΫΒ”ξ
ΔήCO2Ρή≤ζ…ζΈ¬ “–ß”ΠΘ§CO“Ή”κ―Σ“Κ÷–ΒΡ―ΣΚλΒΑΑΉΫαΚœ“ΐΤπ÷–ΕΨ
ΔίCO2Ω…”Οά¥ΟπΜπΘ§COΩ…”ΟΉς»ΦΝœ
…œ ωΥΒΖ®÷–’ΐ»ΖΒΡ «Θ®__________Θ©Θ°
a.ΔΌΔΎΔέ b. ΔΎΔέΔή c.ΔΎΔήΔί d. ΔΌΔέΔί
Θ®3Θ©“‘œ¬ «≥Θ”ΟΒΡ Β―ιΉΑ÷ΟΆΦΘΚ

ΔΌ ’Φ·Ρ≥ΤχΧε÷ΜΡή≤…”ΟDΉΑ÷ΟΘ§”…¥ΥΆΤ≤βΗΟΤχΧεΨΏ”–ΒΡ–‘÷ ________Θ°
ΔΎ–¥≥ω Β―ι “”ΟAΉΑ÷Ο÷Τ»Γ―θΤχΒΡΜ·―ßΖΫ≥Χ Ϋ________Θ°
Δέ”ΟΉΑ÷ΟC÷Τ»ΓΤχΧεΘ§‘ΎΖ¥”ΠΙΐ≥Χ÷–Θ§”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙήΘ§Ιΐ“ΜΜαΖ¥”ΠΨΆΜαΆΘ÷ΙΘ§Τδ‘≠“ρ « ≤Ο¥_________ ΘΩ
ΔήΕΰ―θΜ·ΧΦ”κ≥Έ«ε ·Μ“Υ°Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ________Θ°»τΫΪCO2Ά®»κEΉΑ÷ΟΈ¥Ω¥ΒΫ”Π”–ΒΡΜκΉ«œ÷œσΘ§Τδ‘≠“ρ « ≤Ο¥ΘΩ______
Θ®4Θ©≥Τ»Γ5g ·Μ“ ·Θ®‘”÷ ≤Μ≤ΈΦ”Ζ¥”ΠΘ©Ζ≈»κ…’±≠÷–Θ§œρΤδ÷–Φ”»κΉψΝΩœΓ―ΈΥαΘ§ΫΪΖ¥”Π…ζ≥…ΒΡΤχΧε»Ϊ≤ΩΆ®»κ Δ”–ΉψΝΩ≥Έ«ε ·Μ“Υ°ΒΡ…’±≠÷–Θ®ΤχΧε»Ϊ≤Ω±ΜΈϋ ’Θ©Θ§Ζ¥”ΠΫα χΚσ≥ΤΝΩ…’±≠÷–Έο÷ ΒΡ÷ ΝΩ‘ωΦ”ΝΥ1.76gΘ° ‘ΦΤΥψ ·Μ“ ·÷–Κ§”–‘”÷ ΒΡ÷ ΝΩΖ÷ ΐΘ°______
ΓΨ¥πΑΗΓΩΧΦ‘≠Ή”ΒΡ≈≈Ν–ΖΫ Ϋ≤ΜΆ§ C ΟήΕ»±»Ω’Τχ–ΓΘ§Ρή»ή”ΎΥ°ΜρΡήΚΆΥ°Ζ¥”Π 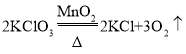 ”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙή ±Θ§ΤχΧε≤ΜΡήΒΦ≥ωΘ§Τχ―Ι‘ω¥σΘ§Α―“ΚΧε―Ι»κ≥ΛΨ±¬©ΕΖΘ§ Ι“ΚΧεΚΆΙΧΧεΖ÷άκΘ§Ζ¥”ΠΆΘ÷Ι Ca(OH)2+CO2=CaCO3
”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙή ±Θ§ΤχΧε≤ΜΡήΒΦ≥ωΘ§Τχ―Ι‘ω¥σΘ§Α―“ΚΧε―Ι»κ≥ΛΨ±¬©ΕΖΘ§ Ι“ΚΧεΚΆΙΧΧεΖ÷άκΘ§Ζ¥”ΠΆΘ÷Ι Ca(OH)2+CO2=CaCO3![]() +H2O ≥Έ«ε ·Μ“Υ°“―Ψ≠Άξ»Ϊ±δ÷ 20%
+H2O ≥Έ«ε ·Μ“Υ°“―Ψ≠Άξ»Ϊ±δ÷ 20%
ΓΨΫβΈωΓΩ
Θ®1Θ©ΫπΗ’ ·ΚΆ ·ΡΪ «ΝΫ÷÷≥ΘΦϊΒΡΧΦΒΞ÷ Θ§ΤδΈοάμ–‘÷ œύ≤νΚή¥σΒΡ‘≠“ρ «ΧΦ‘≠Ή”ΒΡ≈≈Ν–ΖΫ Ϋ≤ΜΆ§ΘΜ
Θ®2Θ©ΔΌ»ΐ÷÷Έο÷ ΕΦΚ§”–ΧΦ‘ΣΥΊΘ§ΧΦΚΆ“Μ―θΜ·ΧΦΡήΙΜ»Φ…’Θ§Εΰ―θΜ·ΧΦ≤ΜΡή»Φ…’Θ§ΗΟ―ΓœνΥΒΖ®≤Μ’ΐ»ΖΘΜ
ΔΎ![]() ΓΔ
ΓΔ![]() ΕΦ «ΟΜ”–―’…ΪΓΔΟΜ”–ΤχΈΕΒΡΤχΧεΘ§ΗΟ―ΓœνΥΒΖ®’ΐ»ΖΘΜ
ΕΦ «ΟΜ”–―’…ΪΓΔΟΜ”–ΤχΈΕΒΡΤχΧεΘ§ΗΟ―ΓœνΥΒΖ®’ΐ»ΖΘΜ
Δέ![]() Ω…”Ο”ΎΙβΚœΉς”ΟΘ§
Ω…”Ο”ΎΙβΚœΉς”ΟΘ§![]() ≤ΜΡή”Ο”Ύ»ΥΙΛΫΒ”ξΘ§ΗΟ―ΓœνΥΒΖ®≤Μ’ΐ»ΖΘΜ
≤ΜΡή”Ο”Ύ»ΥΙΛΫΒ”ξΘ§ΗΟ―ΓœνΥΒΖ®≤Μ’ΐ»ΖΘΜ
Δή![]() Ρή≤ζ…ζΈ¬ “–ß”ΠΘ§
Ρή≤ζ…ζΈ¬ “–ß”ΠΘ§![]() “Ή”κ―Σ“Κ÷–ΒΡ―ΣΚλΒΑΑΉΫαΚœ“ΐΤπ÷–ΕΨΘ§ΗΟ―ΓœνΥΒΖ®’ΐ»ΖΘΜ
“Ή”κ―Σ“Κ÷–ΒΡ―ΣΚλΒΑΑΉΫαΚœ“ΐΤπ÷–ΕΨΘ§ΗΟ―ΓœνΥΒΖ®’ΐ»ΖΘΜ
Δί![]() Ω…”Οά¥ΟπΜπΘ§
Ω…”Οά¥ΟπΜπΘ§![]() Ω…”ΟΉς»ΦΝœΘ§ΗΟ―ΓœνΥΒΖ®’ΐ»ΖΘΜ
Ω…”ΟΉς»ΦΝœΘ§ΗΟ―ΓœνΥΒΖ®’ΐ»ΖΘΜ
Θ®3Θ©ΔΌ ’Φ·Ρ≥ΤχΧε÷ΜΡή≤…”Ο![]() ΉΑ÷ΟΘ§”…¥ΥΆΤ≤βΗΟΤχΧεΨΏ”–ΒΡ–‘÷ «ΟήΕ»±»Ω’Τχ–ΓΘ§Ρή»ή”ΎΥ°ΜρΡήΚΆΥ°Ζ¥”ΠΘΜ
ΉΑ÷ΟΘ§”…¥ΥΆΤ≤βΗΟΤχΧεΨΏ”–ΒΡ–‘÷ «ΟήΕ»±»Ω’Τχ–ΓΘ§Ρή»ή”ΎΥ°ΜρΡήΚΆΥ°Ζ¥”ΠΘΜ
ΔΎ Β―ι “”Ο![]() ΉΑ÷Ο÷Τ»Γ―θΤχ ±Θ§”…”Ύ ‘ΙήΩΎΟΜ”–»ϊ“ΜΆ≈ΟόΜ®Θ§”ΠΗΟ «άϊ”Ο¬»ΥαΦΊΚΆΕΰ―θΜ·ΟΧ÷Τ»Γ―θΤχΘ§¬»ΥαΦΊ‘ΎΕΰ―θΜ·ΟΧ¥ΏΜ·Ής”Οœ¬ ή»»Ζ÷Ϋβ…ζ≥…¬»Μ·ΦΊΚΆ―θΤχΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ
ΉΑ÷Ο÷Τ»Γ―θΤχ ±Θ§”…”Ύ ‘ΙήΩΎΟΜ”–»ϊ“ΜΆ≈ΟόΜ®Θ§”ΠΗΟ «άϊ”Ο¬»ΥαΦΊΚΆΕΰ―θΜ·ΟΧ÷Τ»Γ―θΤχΘ§¬»ΥαΦΊ‘ΎΕΰ―θΜ·ΟΧ¥ΏΜ·Ής”Οœ¬ ή»»Ζ÷Ϋβ…ζ≥…¬»Μ·ΦΊΚΆ―θΤχΘ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ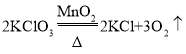 ΘΜ
ΘΜ
Δέ”ΟΉΑ÷Ο![]() ÷Τ»ΓΤχΧεΘ§‘ΎΖ¥”ΠΙΐ≥Χ÷–Θ§”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙήΘ§Ιΐ“ΜΜαΖ¥”ΠΨΆΜαΆΘ÷ΙΘ§Τδ‘≠“ρ «ΘΚ”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙή ±Θ§ΤχΧε≤ΜΡήΒΦ≥ωΘ§Τχ―Ι‘ω¥σΘ§Α―“ΚΧε―Ι»κ≥ΛΨ±¬©ΕΖΘ§ Ι“ΚΧεΚΆΙΧΧεΖ÷άκΘ§Ζ¥”ΠΆΘ÷ΙΘΜ
÷Τ»ΓΤχΧεΘ§‘ΎΖ¥”ΠΙΐ≥Χ÷–Θ§”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙήΘ§Ιΐ“ΜΜαΖ¥”ΠΨΆΜαΆΘ÷ΙΘ§Τδ‘≠“ρ «ΘΚ”ΟΒ·Μ…Φ–Φ–ΉΓΒΦΙή…œΒΡœπΤΛΙή ±Θ§ΤχΧε≤ΜΡήΒΦ≥ωΘ§Τχ―Ι‘ω¥σΘ§Α―“ΚΧε―Ι»κ≥ΛΨ±¬©ΕΖΘ§ Ι“ΚΧεΚΆΙΧΧεΖ÷άκΘ§Ζ¥”ΠΆΘ÷ΙΘΜ
ΔήΕΰ―θΜ·ΧΦ”κ≥Έ«ε ·Μ“Υ°Ζ¥”Π…ζ≥…ΧΦΥαΗΤ≥ΝΒμΚΆΥ°Θ§Ζ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ![]() ΘΜ
ΘΜ
»τΫΪ![]() Ά®»κ
Ά®»κ![]() ΉΑ÷ΟΈ¥Ω¥ΒΫ”Π”–ΒΡΜκΉ«œ÷œσΘ§Τδ‘≠“ρ «≥Έ«ε ·Μ“Υ°“―Ψ≠Άξ»Ϊ±δ÷ ΘΜ
ΉΑ÷ΟΈ¥Ω¥ΒΫ”Π”–ΒΡΜκΉ«œ÷œσΘ§Τδ‘≠“ρ «≥Έ«ε ·Μ“Υ°“―Ψ≠Άξ»Ϊ±δ÷ ΘΜ
Θ®4Θ©…η ·Μ“ ·÷–Κ§”–ΒΡΧΦΥαΗΤΒΡ÷ ΝΩΈΣ![]() Θ§
Θ§
ΗυΨίΧβ“βΩ…“‘÷ΣΒά…’±≠÷–‘ωΦ”ΒΡ÷ ΝΩΦ¥ΈΣ…ζ≥…ΒΡΕΰ―θΜ·ΧΦΒΡ÷ ΝΩΘ§Φ¥…ζ≥…Εΰ―θΜ·ΧΦ÷ ΝΩ «![]() Θ§
Θ§

![]() Θ§
Θ§
![]() Θ§
Θ§
·Μ“ ·÷–‘”÷ ΒΡ÷ ΝΩΖ÷ ΐΈΣΘΚ![]() Θ§
Θ§
¥πΘΚ ·Μ“ ·÷–‘”÷ ΒΡ÷ ΝΩΖ÷ ΐΈΣ![]() ΓΘ
ΓΘ


 ΨΪ”ΔΩΎΥψΩ®œΒΝ–¥πΑΗ
ΨΪ”ΔΩΎΥψΩ®œΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
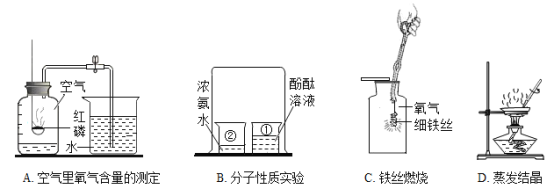
ΓΨΧβΡΩΓΩ Β―ι «―ßœΑΜ·―ßΒΡ÷Ί“ΣΆΨΨΕΘ§œ¬Ν– Β―ι≤ΌΉς¥μΈσΒΡ «Θ® Θ©
A. 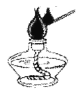 Βψ»ΦΨΤΨΪΒΤB.
Βψ»ΦΨΤΨΪΒΤB. 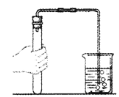 Φλ≤ιΉΑ÷ΟΤχΟή–‘
Φλ≤ιΉΑ÷ΟΤχΟή–‘
C. 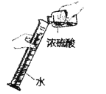 œΓ Ά≈®ΝρΥαD.
œΓ Ά≈®ΝρΥαD. 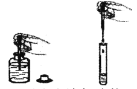 Έϋ»ΓΚΆΒΈΦ”“ΚΧε
Έϋ»ΓΚΆΒΈΦ”“ΚΧε
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ“‘œ¬ «≥θ÷–Μ·―ßΒΡ“Μ–©Μυ±Ψ Β―ι:
(1)A Β―ι÷–Ω…»ΦΈο”Π»ΓΙΐΝΩΒΡ‘≠“ρ «_______________________ΘΜ
(2)B Β―ιΥΒΟςΖ÷Ή”ΨΏ”–ΒΡ–‘÷ «_______________________ΘΜ
(3)C Β―ιΑ¥’’ΆΦ Ψ Β―ι,»ί“ΉΒΦ÷¬ΒΡ≤ΜΝΦΚσΙϊ «_______________________ΘΜ
(4)D Β―ι÷–≤ΘΝßΑτΒΡΉς”Ο «_______________________ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
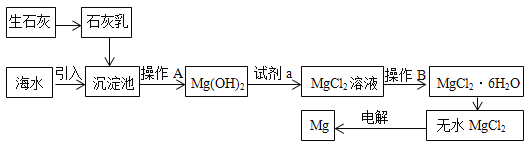
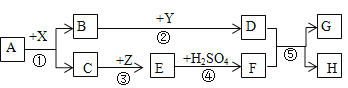
ΓΨΧβΡΩΓΩΘ®IΘ©Ϋπ τΟΨΙψΖΚ”Π”Ο”Ύ…ζΜνΓΔ…ζ≤ζΚΆΙζΖάΙΛ“ΒΓΘ¥”ΚΘΥ°Μρ¬±Υ°÷–Χα»ΓΟΨΒΡΝς≥Χ»γœ¬ΘΚ
ΔΌ≤ΌΉςAΒΡΟϊ≥Τ «_______Θ§≤ΌΉςBΒΡΟϊ≥Τ «__________ΓΘ
ΔΎ ‘ΦΝaΒΡΜ·―ß ΫΈΣ__________ΘΜΈόΥ°MgCl2ΒγΫβΖ¥”ΠΚσΒΡΝμ“Μ≤ζΈο «________ΓΘ
Θ®IIΘ©AΓΪHΕΦ «≥θ÷–Μ·―ß≥ΘΦϊΈο÷ ΓΘΤδ÷–AΓΔBΒΡΉι≥…‘ΣΥΊœύΆ§Θ§«“≥ΘΈ¬œ¬ΕΦ «Έό…Ϊ“ΚΧεΘΜH «άΕ…Ϊ≥ΝΒμΓΘXΓΔYΕΦ «―θΜ·ΈοΘ§Τδ÷–X «ΚΎ…ΪΖέΡ©Ή¥ΙΧΧεΘ§Y≥Θ”ΟΉς ≥ΤΖΗ…‘οΦΝΘ§Z «Κλ…ΪΙΧΧεΓΘΥϋΟ«÷°Φδ”–»γΆΦΉΣΜ·ΙΊœΒΓΘ
ΗυΨί…œ ω–≈œΔΘ§ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©AΒΡΜ·―ß ΫΈΣ ______ ΘΜA‘Ύ“ΫΝΤ…œ≥Θ”ΟΉς_________ ΓΘ
Θ®2Θ© Ζ¥”ΠΔΎ÷–ΑιΥφΉ≈______Θ®ΧνΓΑΖ≈»»Γ±ΜρΓΑΈϋ»»Γ±Θ©œ÷œσΘΜZΒΡ“Μ÷÷”ΟΆΨ «_____ΓΘ
Θ®3Θ©Ζ¥”ΠΔίΒΡΜ·―ßΖΫ≥Χ ΫΈΣ _____ ΘΜ‘ΎΖ¥”ΠΔΌΔΎΔέΔήΔί÷– τ”ΎΜ·ΚœΖ¥”ΠΒΡ”–_______ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬ΆΦ «Έ“ΙζΕ‘ά§Μχ¥Πάμ”κΉέΚœάϊ”ΟΒΡΙΛ“’Νς≥ΧΆΦΘΚ
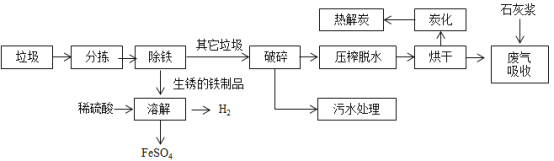
Ή ΝœΘΚΚφΗ…ΓΔΧΩΜ·≤Ϋ÷η…ζ≥…ΒΡΖœΤχ÷–Κ§”–SO2ΓΔHClΒ»ΤχΧεΓΘ
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)»»ΫβΧΩ‘ΎΙΛ“Β…œΩ…“‘…ζ≤ζΜν–‘ΧΩΘ§‘ΎΈέΥ°¥Πάμ÷–Μν–‘ΧΩΒΡΉς”Ο «_________
(2)Νς≥ΧΆΦ÷–≤ζ…ζH2ΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_______Θ§ΖœΤχΈϋ ’≤Ϋ÷η÷–Θ§ ·Μ“Ϋ§ΒΡΉς”Ο «__________________
(3) »ήΫβ≤Ϋ÷η÷–ΜΙΖΔ…ζΝΥ»γœ¬Ζ¥”ΠΘΚΔΌFe2O3+3H2SO4=Fe2(SO4)3+3H2OΔΎFe+Fe2(SO4)3=3FeSO4Θ§ Ήν÷’ΥυΒΟFeSO4»ή“ΚΒΡ―’…Ϊ «_________
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–”–ΙΊΧΦΚΆΧΦΒΡ―θΜ·ΈοΒΡΥΒΖ®÷–Θ§’ΐ»ΖΒΡ «Θ® Θ©
A.ΫπΗ’ ·ΓΔ ·ΡΪΚΆ![]() ΕΦ «”…ΧΦ‘≠Ή”ΙΙ≥…ΒΡΒΞ÷
ΕΦ «”…ΧΦ‘≠Ή”ΙΙ≥…ΒΡΒΞ÷
B.“Μ―θΜ·ΧΦΚΆΕΰ―θΜ·ΧΦΩ…“‘”ΟΉœ…ΪΒΡ ·»ο»ή“ΚΦχ±π
C.ΧΦΚΆ“Μ―θΜ·ΧΦ‘Ύ“ΜΕ®ΧθΦΰœ¬ΕΦΡή”κ―θΜ·Ά≠Ζ¥”Π÷ΟΜΜ≥ωΆ≠
D.Ω’Τχ÷–“Μ―θΜ·ΧΦΜρΕΰ―θΜ·ΧΦΚ§ΝΩΙΐΗΏΕΦΜαΈΘΦΑ»ΥΒΡ…ζΟϋΘ§“ρΈΣΥϋΟ«ΕΦ «ΨγΕΨ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩœ¬Ν–ΥΒΖ®¥μΈσΒΡ «Θ®ΓΓΓΓΘ©
A.≈δ÷Ο200g 5%ΒΡ¬»Μ·ΡΤ»ή“Κ–η“ΣΥ°ΒΡ÷ ΝΩ «190g
B.”ΟΖ ‘μΥ°Ω…“‘«χΖ÷”≤Υ°ΚΆ»μΥ°
C.œ»”ΟΥ°»σ Σ pH ‘÷ΫΘ§‘Ό≤βΕ®»ή“ΚΒΡ pH
D. Σ“¬Ζΰ‘Ύ―τΙβœ¬Η…ΒΟΩλΘ§ «“ρΈΣΈ¬Ε»…ΐΗΏΘ§Υ°Ζ÷Ή”‘ΥΕ·ΥΌ¬ Φ”Ωλ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ Β―ι “÷Τ±ΗΕΰ―θΜ·ΧΦΘ§≤Δ“Σ«σΡήΥφ ±ΩΊ÷ΤΖ¥”ΠΫχ––ΜρΆΘ÷ΙΘ§“‘œ¬ΖϊΚœΗΟΧθΦΰΒΡΖΔ…ζΉΑ÷Ο «Θ®Θ©
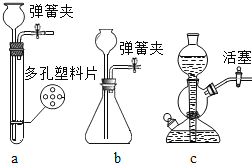
A.![]() ΓΔ
ΓΔ![]() ΓΔ
ΓΔ![]() B.
B.![]() ΓΔ
ΓΔ![]()
C.![]() ΓΔ
ΓΔ![]() D.
D.![]() ΓΔ
ΓΔ![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚ≥θ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
ΓΨΧβΡΩΓΩ÷Ϋ’≈÷–Κ§”– ≤Ο¥‘ΣΥΊΘΩ–ΓΟςΚΆ–ΓΝΝΫχ––ΝΥΧΫΨΩΘ°
[Χα≥ωΈ Χβ]÷Ϋ’≈÷–Κ§”– ≤Ο¥‘ΣΥΊΡΊΘΩ
[≤¬œκΦΌ…η]÷Ϋ’≈÷–Ω…ΡήΚ§”–ΧΦ‘ΣΥΊΚΆ«β‘ΣΥΊΘ°
[≤ι‘ΡΉ Νœ]ΔΌ÷Ϋ’≈»Φ…’ΚσΘ§»γΙϊ…ζ≥…Έο÷–Κ§”–ΧΦ‘ΣΥΊΓΔ«β‘ΣΥΊΘ§‘ρΗΟ÷Ϋ’≈÷–“ΜΕ®Κ§ΧΦ‘ΣΥΊΓΔ«β‘ΣΥΊΘΜ
ΔΎΕΰ―θΜ·ΧΦΤχΧεΩ…“‘ Ι≥Έ«εΒΡ ·Μ“Υ°±δΜκΉ«Θ°
[ Β―ιΧΫΨΩ]
Θ®1Θ©“«ΤςΚΆ”ΟΤΖΘΚάδΕχΗ…‘οΒΡ…’±≠ΓΔά·÷ρΓΔΜπ≤ώΓΔ≥Έ«εΒΡ ·Μ“Υ°Θ°
Θ®2Θ© Β―ιΙΐ≥ΧΘΚ
Β―ι≤Ϋ÷η | Β―ιœ÷œσ | Β―ιΫα¬έ |
ΔΌ_______ | _______ | ”…≤Ϋ÷ηΔΌΩ…÷Σ÷Ϋ’≈÷–“ΜΕ®Κ§”– |
ΔΎ_______ | _______ | ”…≤Ϋ÷ηΔΎΩ…÷Σ÷Ϋ’≈÷–“ΜΕ®Κ§”–ΧΦ‘ΣΥΊ |
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com