
����Ŀ��������ԭ��Ӧ��һ����Ҫ�ķ�Ӧ���밴Ҫ�����������Ŀ��
(1)�Է�ӦNH3+O2����NO+H2O(δ��ƽ)�����������õ����ŷ��������ת�Ƶķ������Ŀ�� ___________________________________________________________���÷�Ӧ�У�________�ǻ�ԭ����________�ǻ�ԭ�������ԭ��������________��
(2)��һ�������£���Ӧ2NH3+3CuO![]() 3Cu+N2+3H2O��˳�����У��Դ˷�Ӧ�ķ�����������________��
3Cu+N2+3H2O��˳�����У��Դ˷�Ӧ�ķ�����������________��
���÷�Ӧ���û���Ӧ
����Ӧ��NH3������ΪN2
���ڷ�Ӧ�������˽���ͭ�Ļ�ԭ��
���ڷ�Ӧ��ÿ����1 mol H2Oת��1 mol����
(3)�ڷ�Ӧ2H2S+SO2![]() 3S+2H2O�б�������Ԫ���뱻��ԭ��Ԫ�ص�������Ϊ ________��
3S+2H2O�б�������Ԫ���뱻��ԭ��Ԫ�ص�������Ϊ ________��
���𰸡�(1)![]()
(2)��
(3)2��1
��������(1)�ɻ��ϼ۱仯��֪��NH3�ǻ�ԭ����O2����������NO���������������ǻ�ԭ���H2O�ǻ�ԭ���
(2)��Ӧ����û�е��ʲ��룬��һ�������û���Ӧ����������Ӧ��NԪ�ػ��ϼ���3���ߵ�0����NH3������ΪN2������ȷ��NH3�ڷ�Ӧ�����ֻ�ԭ�ԣ��������ɻ��ϼ۱仯��֪���÷�Ӧת��6e����ÿ����1 mol H2Oת��2 mol���ӣ�������
(3)�ڷ�Ӧ��H2S��SԪ�ر�������SO2��SԪ�ر���ԭ������������֮��Ϊ2��1��


 ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ����ͼ�е缫a��b�ֱ�ΪAg�缫��Pt�缫���缫c��d����ʯī�缫��ͨ��һ��ʱ�����c��d�����Ϲ��ռ���336 mL(��״��)���塣�ش�
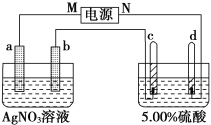
��1��ֱ����Դ�У�MΪ________����
��2��Pt�缫�����ɵ�������________��������Ϊ______________________g��
��3����Դ����ĵ��ӣ������ʵ�����缫b��c��d�ֱ����ɵ����ʵ����ʵ���֮��Ϊ2��________��________��________��
��4��AgNO3��Һ��Ũ��________(�������С�����䡱����ͬ)��AgNO3��Һ��pH________�������Ũ��________�������pH________��
��5�������������������5.00%��Ϊ5.02%����ԭ��5.00%������________g��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��С����ʵ���ҽ�������ʵ�飺���ĸ���ȫ��ͬ�Ŀ��ı���Ƥ��ֱ�����ĸ�ʢ���ܶ�Ϊ��(g/cm3)��ʳ��ˮ���ձ��У�����������λ����ͼ��ʾ��Ȼ������ˮ���ܶȾ�Ϊ��(g/cm3)��������Һ(CuSO4��AgNO3��ϡ����)�ֱ���������ĸ��ձ��С��ش��������⣺
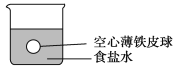
(1)����ˮʱ��������������____________________________________________��
(2)����ϡ����ʱ������������(�ٶ�������Ӧ��������Ƥ�������)_____________________��������Ӧ�����ӷ���ʽ��____________________________________________��
(3)����CuSO4��Һʱ������������___________________________________________________��
������Ӧ�����ӷ���ʽ��______________________________________________��
(4)����AgNO3��Һʱ������������_________________________________��������Ӧ�����ӷ���ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ������ͼ��ʾ�ļס��ҡ������־��壺
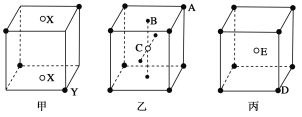
�����
(1)����Ļ�ѧʽ(XΪ������)Ϊ________��
(2)�Ҿ�����A��B��C�������ӵĸ�������________��
(3)��������ÿ��D��Χ���E�ĸ�����________����
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��һ���¶��£��������ˮϡ��������Һ�ĵ���������ͼ��ʾ����ش�
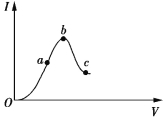
��1��O��Ϊʲô������___________________________��
��2��a��b��c����c(H��)�ɴ�С��˳����______________________________________________��
��3��a��b��c�����д���ĵ���̶����ĵ���________�㡣
��4����ʹc����Һ�е�c(CH3COO)��ߣ��ɲ�ȡ�Ĵ�ʩ��________(����)��
A������ B���Ӻ�ϡ��NaOH��Һ C���ӹ���KOH
D����ˮ E���ӹ���CH3COONa F����п��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ��(1)�ж�������������Ӧ�������ܷ���룬��˵�����ɡ�
��Һ̬HCl��__________________�����ɣ�_________________________________��
������״̬�µ�NaCl��__________________�����ɣ�_________________________________��
�������ۻ���ĵ�������__________________�����ɣ�_________________________________��
������KOH��__________________�����ɣ�________________________________��
(2)д������������ˮ��Һ�еĵ��뷽��ʽ��
HCl��__________________________________________________________________��
H2SO4��________________________________________________________________��
Ca(OH)2��______________________________________________________________��
KOH��________________________________________________________________��
NH4NO3��_____________________________________________________________��
KAl(SO4)2��____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ�����������������������ǵ�����������ʡ�
(1)�γ������ԭ��֮һ�ɼ�ʾ���£�
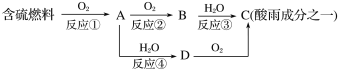
�ش��������⣺
�������pH________(���������������=��)5.6��
��D���ʵĻ�ѧʽΪ____________��
����Ӧ���Ļ�ѧ����ʽΪ_________________________________________��
(2)��һ�������°��������������������ת��Ϊ����Ⱦ�����ʡ�д�������Ͷ���������һ�������·�Ӧ�Ļ�ѧ����ʽ��__________________����Ӧ����������____________����ԭ����_______________��
(3)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�
NO2+NO+2NaOH===2NaNO2+H2O��2NO2+2NaOH===NaNO2+NaNO3+H2O
����V LijNaOH��Һ����ȫ����n mol NO2��m mol NO��ɵĴ�����Ⱦ�
�������ռ���Һ�����ʵ���Ũ������Ϊ________ mol��L1��
����������Һ��c(![]() )��c(
)��c(![]() )=1��9����ԭ���������NO2��NO�����ʵ���֮��n��m=______��
)=1��9����ԭ���������NO2��NO�����ʵ���֮��n��m=______��
���ú�n��m�Ĵ���ʽ��ʾ������Һ��![]() ��
��![]() Ũ�ȵı�ֵc(
Ũ�ȵı�ֵc(![]() )��c(
)��c(![]() )=________��
)=________��
�鿴�𰸺ͽ���>>
��Ŀ�����е��� ��Դ�� ���ͣ�
����Ŀ���±���ʾ�Ǽ���������ʵĵ���ƽ�ⳣ����ij���ܵ���ʵ��ܶȻ�Ksp(25 ��)��
����� | ƽ�ⷽ��ʽ | ����ƽ�ⳣ�� | Ksp |
CH3COOH | CH3COOH | 1.76��105 | |
H2CO3 | H2CO3 HC | Ka1=4.31��107 Ka2=5.61��1011 | |
C6H5OH | C6H5OH | 1.1��1010 | |
H3PO4 | H3PO4
| Ka1=7.52��103 Ka2=6.23��108 Ka3=2.20��1013 | |
NH3��H2O | NH3��H2O | 1.76��105 | |
BaSO4 | BaSO4(s) | 1.07��1010 |
�ش�����������
��1�����ϱ�����������CH3COOH����![]() ����C6H5OH����
����C6H5OH����![]() ���ɿ������������ǵ�������ǿ������˳��Ϊ (����)��
���ɿ������������ǵ�������ǿ������˳��Ϊ (����)��
��2��25 ��ʱ�������������Ũ�ȵ�CH3COOH��Һ�Ͱ�ˮ��������Һ����c(CH3COO) (�>����=����<��)c(![]() )��
)��
��3��25 ��ʱ����10 mL 0.01 mol��L1������Һ�еμ�V mL 0.01 mol��L1��ˮ������˵����ȷ���� (�����)��
A�������ҺpH>7����V��10
B�������ҺpH<7����c(![]() )>c(C6H5O)>c(H+)>c(OH)
)>c(C6H5O)>c(H+)>c(OH)
C��V=10ʱ�����Һ��ˮ�ĵ���̶�С��10 mL 0.01 mol��L1������Һ��ˮ�ĵ���̶�
D��V=5ʱ��2c(NH3��H2O)+2c(![]() )=c(C6H5O)+c(C6H5OH)
)=c(C6H5O)+c(C6H5OH)
��4����ͼ��ʾΪij�¶�ʱBaSO4�ij����ܽ�ƽ������������˵������ȷ���� (�����)��
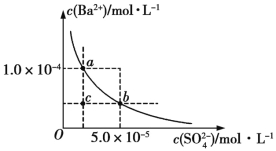
A������Na2SO4��ʹ��Һ��a���Ϊb��
B���������Ϸ�����(��������)����һ��ʱ������BaSO4��������
C�������ܼ�����ʹ��Һ��c���Ϊ������a��b֮���ijһ��(����a��b)
D�������¶�����ʹ��Һ��b���Ϊc��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com