
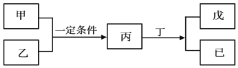
°æƒø°øW°¢X°¢Y°¢ZŒ™‘≠◊”–Ú ˝“¿¥Œ‘ˆ¥Ûµƒ∂Ã÷Ð∆⁄÷˜◊‘™Àÿ£¨∆‰÷–Y‘™Àÿ‘⁄Õ¨÷Ð∆⁄÷–¿Î◊”∞Îæ∂◊Ó–°£ªº◊°¢““∑÷± «‘™ÀÿY°¢Zµƒµ•÷ £ª±˚°¢ ∂°°¢ŒÏ «”…W°¢X°¢Y°¢Z‘™Àÿ◊È≥…µƒ∂˛‘™ªØ∫œŒÔ£¨≥£Œ¬œ¬∂°Œ™“∫裪ŒÏŒ™À·–‘∆¯Ã£¨≥£Œ¬œ¬0.01 ![]() ŒÏ»Ð“∫µƒpH¥Û”⁄2°£…œ ˆŒÔ÷ ◊™ªØπÿœµ»ÁÕºÀ˘ æ°£œ¬¡–Àµ∑®’˝»∑µƒ «( )
ŒÏ»Ð“∫µƒpH¥Û”⁄2°£…œ ˆŒÔ÷ ◊™ªØπÿœµ»ÁÕºÀ˘ æ°£œ¬¡–Àµ∑®’˝»∑µƒ «( )
A.‘≠◊”∞Îæ∂£∫Z>Y>X>W
B.W∫ÕX–Œ≥…µƒªØ∫œŒÔº»ø…ƒÐ∫¨”–º´–‘º¸“≤ø…ƒÐ∫¨”–∑«º´–‘º¸
C.W°¢X°¢Y°¢Z≤ªø…ƒÐÕ¨¥Ê”⁄“ª÷÷¿Î◊”ªØ∫œŒÔ÷–
D.±»ΩœX°¢Z∑«Ω Ù–‘«ø»ı ±£¨ø…±»Ωœ∆‰◊Ó∏þº€—ıªØŒÔ∂‘”¶µƒÀƪ،ԵƒÀ·–‘
°æ¥∞∏°øB
°æΩ‚Œˆ°ø
W°¢X°¢Y°¢ZŒ™‘≠◊”–Ú ˝“¿¥Œ‘ˆ¥Ûµƒ∂Ã÷Ð∆⁄÷˜◊‘™Àÿ£¨±˚°¢∂°°¢ŒÏ «”…W°¢X°¢Y°¢Z‘™Àÿ◊È≥…µƒ∂˛‘™ªØ∫œŒÔ£¨≥£Œ¬œ¬∂°Œ™“∫裨∂°Œ™H2O£¨WŒ™«‚‘™Àÿ°£º◊°¢““∑÷± «‘™ÀÿY°¢Zµƒµ•÷ £¨∆‰÷–Y‘™Àÿ‘⁄Õ¨÷Ð∆⁄÷–¿Î◊”∞Îæ∂◊Ó–°£¨º◊”Γ“ªØ∫œ…˙≥…±˚£¨±˚”⁄ÀÆ∑¥”¶…˙≥…ŒÏ£¨ŒÏŒ™À·–‘∆¯Ã£¨≥£Œ¬œ¬0.01mol°§L-1ŒÏ»Ð“∫µƒpH¥Û”⁄2£¨ŒÏ»Ð“∫Œ™»ıÀ·£¨”¶ «Al2S3”ÎÀÆ∑¥”¶…˙≥…H2S”ÎAl(OH)3£¨π ±˚Œ™Al2S3°¢ŒÏŒ™H2S°¢º∫Œ™Al(OH)3£¨Ω·∫œ‘≠◊”–Ú ˝ø…÷™XŒ™—ı‘™Àÿ°¢YŒ™Al°¢ZŒ™¡Ú‘™Àÿ£¨º◊Œ™O2°¢““Œ™¡Úµ•÷ °£
A£ÆÕ¨÷Ð∆⁄¥”◊ÛµΩ”“‘≠◊”∞Îæ∂ºı–°£¨Õ¨÷˜◊Â◊‘…œ∂¯œ¬‘≠◊”∞Îæ∂‘ˆ¥Û£¨À˘”–‘™Àÿ÷–H‘≠◊”∞Îæ∂◊Ó–°£¨‘≠◊”∞Îæ∂£∫Y£®Al£©£æZ£®S£©£æX£®O£©£æW£®H£©£¨π A¥ÌŒÛ£ª
B£ÆW∫Õ¢˙–Œ≥…µƒªØ∫œŒÔ”–H2O°¢H2O2£¨H2O2÷–º»ø…ƒÐ∫¨”–º´–‘º¸“≤ø…ƒÐ∫¨”–∑«º´–‘º¸£¨π B’˝»∑£ª
C£ÆH°¢O°¢Al°¢Sø…ƒÐÕ¨¥Ê”⁄“ª÷÷¿Î◊”ªØ∫œŒÔ÷–£¨»ÁKAl(SO4)2°§12H2O£¨π C¥ÌŒÛ£ª
D£Æ—ı‘™Àÿ√ª”–◊Ó∏þº€—ıªØŒÔ£¨π D¥ÌŒÛ£ª
π —°B°£


 ‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
‘ƒ∂¡øÏ≥µœµ¡–¥∞∏
| ƒÍº∂ | ∏þ÷–øŒ≥à | ƒÍº∂ | ≥ı÷–øŒ≥à |
| ∏þ“ª | ∏þ“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı“ª | ≥ı“ª√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ∂˛ | ∏þ∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı∂˛ | ≥ı∂˛√‚∑—øŒ≥ÃÕ∆ºˆ£° |
| ∏þ»˝ | ∏þ»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° | ≥ı»˝ | ≥ı»˝√‚∑—øŒ≥ÃÕ∆ºˆ£° |
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡–”–πÿµÁΩ‚÷ »Ð“∫µƒÀµ∑®’˝»∑µƒ «
A.Ω´0.1 mol°§L£≠1 CH3COOH»Ð“∫¥”20 °Ê…˝Œ¬÷¡30 °Ê£¨»Ð“∫÷–c(H+)/c(CH3COOH)ºı–°
B.»ÙNH4Cl»Ð“∫”ÎNH4HSO4»Ð“∫µƒpHœýµ»£¨‘Úc(NH![]() )“≤œýµ»
)“≤œýµ»
C.œÚ—ŒÀ·÷–º”»Î∞±ÀÆ÷¡÷––‘£¨»Ð“∫÷–![]() >1
>1
D.≥£Œ¬œ¬£¨pH£Ω2µƒ¥◊À·»Ð“∫”ÎpH£Ω12µƒNaOH»Ð“∫µ»Ãª˝ªÏ∫œ∫Û£¨»Ð“∫pH£º7
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡–«˙œþ∑÷±±Ì æ‘™Àÿµƒƒ≥÷÷–‘÷ ”Î∫ÀµÁ∫… ˝µƒπÿœµ(ZŒ™∫ÀµÁ∫… ˝£¨YŒ™‘™Àÿµƒ”–πÿ–‘÷ )°£
(1)∞—”Îœ¬√Ê‘™Àÿ”–πÿ–‘÷ œý∑˚µƒ«˙œþ±Í∫≈ÃӻΜý”¶µƒø’∏Ò÷–:
a. 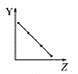 b.
b. 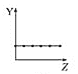 c.
c. 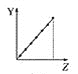 d.
d. 
¢Ÿµ⁄¢ÚA◊‘™Àÿµƒº€µÁ◊” ˝________°£
¢⁄µ⁄»˝÷Ð∆⁄‘™Àÿµƒ◊Ó∏þªØ∫œº€________°£
¢€F-°¢Na+°¢Mg2+°¢Al3+µƒ¿Î◊”∞Îæ∂________°£
(2)‘™ÀÿX°¢Y°¢Z°¢M°¢Næ˘Œ™∂Ã÷Ð∆⁄÷˜◊‘™Àÿ£¨«“‘≠◊”–Ú ˝“¿¥Œ‘ˆ¥Û°£“—÷™Y‘≠◊”◊ÓÕ‚≤„µÁ◊” ˝”Î∫ÀÕ‚µÁ◊”◊Ð ˝÷Ʊ»Œ™3°√4£ªM‘™Àÿ‘≠◊”µƒ◊ÓÕ‚≤„µÁ◊” ˝”εÁ◊”≤„ ˝÷Ʊ»Œ™4°√3£ªN-°¢Z+°¢X+µƒ∞Îæ∂÷Ω•ºı–°£ªªØ∫œŒÔXN≥£Œ¬œ¬Œ™∆¯Ã°£æð¥Àªÿ¥£∫
¢ŸXŒ™___________(√˚≥∆)£¨YŒ™____________(‘™Àÿ∑˚∫≈)£¨Z‘≠◊”Ω·ππ æ“‚ÕºŒ™________________°£
¢⁄Nµƒ◊Ó∏þº€—ıªØŒÔµƒÀƪ،ԵƒªØ—ß ΩŒ™________________°£
¢€Mµƒ◊Ó∏þº€—ıªØŒÔµƒªØ—ß ΩŒ™________________________°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øƒ—ª”∑¢–‘∂˛¡ÚªØÓ„£®TaS2 £©ø…≤…”√»Áœ¬◊∞÷√÷¥ø°£Ω´≤ª¥øµƒTaS2 ∑€ƒ©◊∞»Î Ø”¢πГª∂À£¨≥È’Êø’∫Û“˝»Î ¡øµ‚≤¢∑‚πУ¨÷√”⁄º”»»¬Ø÷–°£∑¥”¶»Áœ¬£∫
TaS2£®g£©+2I2£®g£©![]() TaI4£®g£©+S2£®g£©
TaI4£®g£©+S2£®g£©

œ¬¡–Àµ∑®’˝»∑µƒ « £® £©
A£Æ‘⁄≤ªÕ¨Œ¬∂»«¯”Ú£¨TaI4 µƒ¡ø±£≥÷≤ª±‰
B£Æ‘⁄÷¥øπ˝≥Ã÷–£¨I2 µƒ¡ø≤ª∂œºı…Ÿ
C£Æ‘⁄÷¥øπ˝≥Ã÷–£¨I2µƒ◊˜”√ «Ω´TaS2 ¥”∏þŒ¬«¯◊™“∆µΩµÕŒ¬«¯
D£Æ∏√’˝∑¥”¶µƒ∆Ω∫‚≥£ ˝”ÎTaI4 ∫ÕS2 µƒ≈®∂»≥Àª˝≥…∑¥±»
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø‘⁄“ª√б’»ð∆˜£¨aA(g)![]() bB(g)¥Ô∆Ω∫‚∫Û£¨±£≥÷Œ¬∂»≤ª±‰£¨Ω´»ð∆˜Ãª˝‘ˆº”“ª±∂£¨µ±¥ÔµΩ–¬µƒ∆Ω∫‚ ±£¨Bµƒ≈®∂» «‘≠¿¥µƒ60%£¨‘Ú£® £©
bB(g)¥Ô∆Ω∫‚∫Û£¨±£≥÷Œ¬∂»≤ª±‰£¨Ω´»ð∆˜Ãª˝‘ˆº”“ª±∂£¨µ±¥ÔµΩ–¬µƒ∆Ω∫‚ ±£¨Bµƒ≈®∂» «‘≠¿¥µƒ60%£¨‘Ú£® £©
A.ŒÔ÷ Bµƒ÷ ¡ø∑÷ ˝‘ˆº”¡ÀB.ŒÔ÷ Aµƒ◊™ªØ¬ ºı…Ÿ¡À
C.∆Ω∫‚œÚƒÊ∑¥”¶∑ΩœÚ“∆∂Ø¡ÀD.a£æb
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øƒ≥Œ¬∂»œ¬£¨‘⁄“ª∏ˆ2Lµƒ√б’»ð∆˜÷–£¨º”»Î4mo1A∫Õ2molBΩ¯––»Áœ¬∑¥”¶£∫3A(g)+2B(g)![]() 4C(£ø)+2D(£ø)°£∑¥”¶“ª∂Œ ±º‰∫Û¥ÔµΩ∆Ω∫‚£¨≤‚µ√…˙≥…1.6molC£¨«“∑¥”¶«∞∫Ûµƒ—π«ø÷Ʊ»Œ™5:4£®œýÕ¨µƒŒ¬∂»œ¬≤‚¡ø£©£¨‘Úœ¬¡–Àµ∑®’˝»∑µƒ «
4C(£ø)+2D(£ø)°£∑¥”¶“ª∂Œ ±º‰∫Û¥ÔµΩ∆Ω∫‚£¨≤‚µ√…˙≥…1.6molC£¨«“∑¥”¶«∞∫Ûµƒ—π«ø÷Ʊ»Œ™5:4£®œýÕ¨µƒŒ¬∂»œ¬≤‚¡ø£©£¨‘Úœ¬¡–Àµ∑®’˝»∑µƒ «
A.∏√∑¥”¶µƒªØ—ß∆Ω∫‚≥£ ˝±Ì¥Ô Ω «K=![]()
B.¥À ±£¨Bµƒ∆Ω∫‚◊™ªØ¬ «35%
C.‘ˆ¥Û∏√ÃÂœµµƒ—π«ø£¨∆Ω∫‚œÚ”““∆∂Ø£¨ªØ—ß∆Ω∫‚≥£ ˝‘ˆ¥Û
D.‘ˆº”Cµƒ¡ø£¨Bµƒ∆Ω∫‚◊™ªØ¬ ≤ª±‰
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø£®1£© µ—È≤‚µ√16 gº◊¥º[CH3OH(l)]‘⁄—ı∆¯÷–≥‰∑÷»º…’…˙≥…∂˛—ıªØú∆¯ÃÂ∫Õ“∫èÀÆ ± Õ∑≈≥ˆ363.25 kJµƒ»»¡ø£¨ ‘–¥≥ˆº◊¥º»º…’»»µƒ»»ªØ—ß∑Ω≥Ã Ω£∫___°£
£®2£©¥”ªØ—ߺ¸µƒΩ«∂»∑÷Œˆ£¨ªØ—ß∑¥”¶µƒπ˝≥ÃæÕ «∑¥”¶ŒÔµƒªØ—ߺ¸±ª∆∆ªµ∫Õ…˙≥…ŒÔµƒªØ—ߺ¸µƒ–Œ≥…π˝≥𣓗÷™∑¥”¶£∫N2(g)£´3H2(g)![]() 2NH3(g) ¶§H£ΩakJ°§mol£≠1°£”–πÿº¸ƒÐ ˝æð»Áœ¬£∫
2NH3(g) ¶§H£ΩakJ°§mol£≠1°£”–πÿº¸ƒÐ ˝æð»Áœ¬£∫
ªØ—ߺ¸ | H°™H | N°™H | N°‘N |
º¸ƒÐ(kJ°§mol£≠1) | 436 | 391 | 945 |
‘∏˘æð±Ì÷–À˘¡–º¸ƒÐ ˝æðº∆À„a=____°£
£®3£©“¿æð∏«Àπ∂®¬…ø…“‘∂‘ƒ≥–©ƒ—“‘Õ®π˝ µ—È÷±Ω”≤‚∂®µƒªØ—ß∑¥”¶µƒ∑¥”¶»»Ω¯––Õ∆À„°£
“—÷™£∫C(s£¨ ؃´)£´O2(g)=CO2(g) ¶§H1£Ω£≠393.5kJ°§mol£≠1
2H2(g)£´O2(g)=2H2O(l) ¶§H2£Ω£≠571.6kJ°§mol£≠1
2C2H2(g)£´5O2(g)=4CO2(g)£´2H2O(l) ¶§H3£Ω£≠2599kJ°§mol£≠1
∏˘æð∏«Àπ∂®¬…£¨º∆À„298 K ±”…C(s£¨ ؃´)∫ÕH2(g)…˙≥…1 mol C2H2(g)∑¥”¶µƒ∑¥”¶»»Œ™£∫¶§H£Ω___°£
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°øœ¬¡–Àµ∑®’˝»∑µƒ «£® £©
A.œÚƒ≥»Ð“∫÷–µŒº”…Ÿ¡øAgNO3»Ð“∫£¨”–∞◊…´≥¡µÌ≤˙…˙£¨»Ð“∫÷–“ª∂®∫¨”–Cl-
B.œÚƒ≥»Ð“∫÷–º”»Î“ª∂®¡øµƒœ°¡ÚÀ·£¨≤˙…˙µƒ∆¯Ã π≥Œ«Â ت“ÀƱ‰ªÎ◊«£¨»Ð“∫÷–“ª∂®∫¨”–SO32-ªÚCO32-
C.Ω´◊∆»»µƒƒæÃø”Î≈®œıÀ·∑¥”¶≤˙…˙µƒ∆¯Ãª∫ª∫Õ®»Î≥Œ«Â ت“ÀÆ÷–£¨»Ð“∫√ª”–≥¡µÌ≤˙…˙
D.œÚƒ≥»Ð“∫÷–µŒº”…Ÿ¡ø≈®NaOH»Ð“∫£¨Ω´ ™»Û∫Ï…´ Ø»Ô ‘÷Ω÷√”⁄ ‘πÐø⁄£¨ ‘÷Ω≤ª±‰¿∂£¨Àµ√˜∏√»Ð“∫÷–“ª∂®≤ª∫¨NH4+
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
ø∆ƒø£∫∏þ÷–ªØ—ß ¿¥‘¥£∫ –գ∫
°æƒø°ø∂‘œýÕ¨ŒÔ÷ µƒ¡øµƒ““œ©∫Õ±˚œ©£¨”–œ¬¡–– ˆ£∫¢ŸÃº‘≠◊” ˝÷Ʊ»Œ™ 2£∫3¢⁄«‚‘≠◊” ˝÷Ʊ»Œ™ 2£∫3¢€∫¨Ãºµƒ÷ ¡ø∑÷ ˝œýÕ¨¢Ð∑÷◊”∏ˆ ˝÷Ʊ»Œ™ 1£∫1£Æ∆‰÷–’˝»∑µƒ « (°°°° )
A.¢Ÿ¢ÐB.¢Ÿ¢⁄¢ÐC.¢Ÿ¢⁄¢€D.¢Ÿ¢⁄¢€¢Ð
≤Èø¥¥∞∏∫ÕΩ‚Œˆ>>
π˙º —ß–£”≈—° - ¡∑œ∞≤·¡–±Ì - ‘¡–±Ì
∫˛±± °ª•¡™Õ¯Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®∆Ωî | Õ¯…œ”–∫¶–≈œ¢æŸ±®◊®«¯ | µÁ–≈’©∆≠柱®◊®«¯ | …Ê¿˙ ∑–ÈŒÞ÷˜“”–∫¶–≈œ¢æŸ±®◊®«¯ | …Ê∆Û«÷»®æŸ±®◊®«¯
Œ•∑®∫Õ≤ª¡º–≈œ¢æŸ±®µÁª∞£∫027-86699610 柱®” œ‰£∫58377363@163.com