
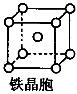
 3Na2O��2Fe��9N2����
3Na2O��2Fe��9N2����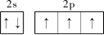 ��2�֣�
��2�֣� HN3+OH?��2�֣�
HN3+OH?��2�֣�
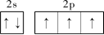 ����3�����ݵȵ�����ĸ����жϣ���N3����Ϊ�ȵ�����ķ���ΪCO2��N2O�����ݼ۲���ӶԻ������ۣ�NO3��������ԭ�������Լ۵��ӣ��ռ乹��Ϊƽ�������Σ���4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ���Ӿ��壻��������Ϊǿ�������Σ����е������ˮ�⣬ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��N3��+H2O
����3�����ݵȵ�����ĸ����жϣ���N3����Ϊ�ȵ�����ķ���ΪCO2��N2O�����ݼ۲���ӶԻ������ۣ�NO3��������ԭ�������Լ۵��ӣ��ռ乹��Ϊƽ�������Σ���4���������ƣ�NaNH2���͵������ƣ�NaN3���ľ�������Ϊ���Ӿ��壻��������Ϊǿ�������Σ����е������ˮ�⣬ˮ��Һ�ʼ��ԣ������ӷ���ʽ��ʾ��ԭ��N3��+H2O HN3+OH������5��N2O�е㣨��88��49�棩��NH3�е㣨��33��34�棩�ͣ�����Ҫԭ���ǰ�����֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ������6���ٵ������к��е�������������1���Ҽ��ͺ�2���м�����Ŀ֮��Ϊ1��2����������Ϊ�������壬���ڵĻ�ѧ������Ϊ����������������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������ȱ�پ����ṹ�����н�����
HN3+OH������5��N2O�е㣨��88��49�棩��NH3�е㣨��33��34�棩�ͣ�����Ҫԭ���ǰ�����֮����������N2O���Ӽ�ֻ���ڷ��»�����������ý�ǿ������6���ٵ������к��е�������������1���Ҽ��ͺ�2���м�����Ŀ֮��Ϊ1��2����������Ϊ�������壬���ڵĻ�ѧ������Ϊ����������������Ϊ���������ѻ����侧����ͼ��ʾ�������߳�Ϊa cm�����������ܶ�Ϊ___________���ú�a��NA�ı���ʽ��ʾ������NAΪ�����ӵ���������ȱ�پ����ṹ�����н�����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 �����幹���� ��
�����幹���� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��H2O | B��CO2 | C��SO2 | D��BeCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
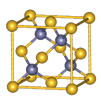
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
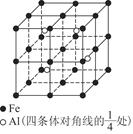
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ������/��kJ��mol��1�� | I1 | I2 | I3 | I4 |
| A | 932 | 1 821 | 15 390 | 21 771 |
| B | 738 | 1 451 | 7 733 | 10 540 |
 ��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ��
��ÿ��COCl2�����ں���________���Ҽ���________���м���������ԭ�Ӳ�ȡ________�ӻ������ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
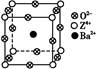
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| A��C3H8��̼ԭ�Ӷ����õ���sp3�ӻ� |
| B��O2��CO2��N2���ǷǼ��Է��� |
| C��ÿ��N2�У�����2���м� |
| D��CO��һ�ֵȵ�����ΪNO�������ĵ���ʽΪ[��N??O��]�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �Ŀռ乹��Ϊ��������,���以Ϊ�ȵ������һ�ַ���Ϊ����������
�Ŀռ乹��Ϊ��������,���以Ϊ�ȵ������һ�ַ���Ϊ���������� 
| A�����Ӽ� | B���Ҽ� | C���м� | D�������E.��λ����F.��������G.���Լ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com