
����Ŀ����֪��1mol�����еĻ�ѧ����Ҫ����436kJ��������1mol�����еĻ�ѧ����Ҫ����498kJ����������ͼ�е�����ͼ���ش��������⣺

��1���ֱ�д���٢ڵ���ֵ��
��____________�� �� ____________ ��
��2������H2O��g���е�1mol H-O���ų�____________kJ��������
��3����֪��H2O��l��= H2O��g�� ��H = +44 kJ��mol��1 ����д����������������ȫȼ������Һ̬ˮ���Ȼ�ѧ����ʽ��_____________________________________��
���𰸡�1370 1852 463 2H2��g��+O2(g) == 2H2O��l����H= ��570kJ/mol
��������
��ͼ���֪2H2��g��+O2��g��=2H2O��g����H=-482kJmol-1����ѧ��Ӧ��ʵ���Ǿɼ��Ķ��Ѻ��¼����γɣ���ѧ������Ҫ�����������γ��¼�Ҫ�ų���������Ӧ��Ϊ��Ӧ����ܼ��ܼ�ȥ��������ܼ��ܣ��Դ˽����⡣
��1�����У���֪��1 mol�����еĻ�ѧ����Ҫ����436 kJ��������1 mol�����еĻ�ѧ����Ҫ����498 kJ ���������Բ�2Ħ��������1Ħ����������Ҫ������Ϊ2��436+498=1370kJ�����и��������غ㣬����֪���ų�������Ϊ1370+482=1852kJ��
��2������H2O��g����1 mol H��O���ų�������Ϊ 1852 /4=463kJ��
��3����ͼ����Ϣ����֪����2H2��g��+O2��g��=2H2O��g����H =-482kJ��mol-1��
����ΪH2O��l��=H2O��g����H = +44 kJ��mol-1������2 mol������������������ȫȼ������Һ̬ˮ���Ȼ�ѧ����Ϊ2H2��g��+O2��g��=2H2O��l����H =-570 kJ��mol-1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����A��Ԫ��(C��Si��Ge��Sn��Pb)���仯�����ڲ��ϵȷ�������Ҫ��;���ش��������⣺
(1) Pb�ļ۲�����Ų�ͼΪ��____________��
(2)GeC14������ԭ�ӵļ۲���Ӷ���Ϊ____________�����ӵ����幹��Ϊ___________��GeC14��ˮ������һ���������һ�������ᣬ�仯ѧ��Ӧ����ʽΪ��________________________________��
(3)��±����![]() �ķе�Ͷ�±��Ǧ
�ķе�Ͷ�±��Ǧ![]() ���۵�����ͼ��ʾ��
���۵�����ͼ��ʾ��

��![]() �ķе���F��CI��Br��I�������ߵ�ԭ����____________��
�ķе���F��CI��Br��I�������ߵ�ԭ����____________��
�ڽ��![]() �ķе��
�ķе��![]() ���۵�ı仯���ɣ����ƶϣ���F��Cl��Br��I����
���۵�ı仯���ɣ����ƶϣ���F��Cl��Br��I����![]() �л�ѧ����������__________(������ǿ������������������������ͬ)��������__________��
�л�ѧ����������__________(������ǿ������������������������ͬ)��������__________��

(4)̼����һ�ֵ���![]() ��������γɵ��³�������������ṹ��ͼ��ʾ���˻�����ɿ�����K�����
��������γɵ��³�������������ṹ��ͼ��ʾ���˻�����ɿ�����K�����![]() �γɵ������������϶�Ͱ������϶֮�У����������϶��ĿΪ_________������һ�ּ����X(���ԭ������ΪM)��
�γɵ������������϶�Ͱ������϶֮�У����������϶��ĿΪ_________������һ�ּ����X(���ԭ������ΪM)��![]() ���γ����ƻ������Xֻ���
���γ����ƻ������Xֻ���![]() �γɵİ������϶��һ�룬�˻�����Ļ�ѧʽΪ��__________���侧������Ϊ1.4nm�������ܶ�Ϊ_________
�γɵİ������϶��һ�룬�˻�����Ļ�ѧʽΪ��__________���侧������Ϊ1.4nm�������ܶ�Ϊ_________![]() (�ú�M�Ͱ���٤��������ֵ
(�ú�M�Ͱ���٤��������ֵ![]() ��ʽ�ӱ�ʾ)
��ʽ�ӱ�ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���α������һ������Ѫ�����ŵĽ�Ѫѹҩ�һ�ֺϳ��α�����м���G�IJ����������£�

��֪���������Ľṹ��ʽΪ![]() ��
��
��ش��������⣺
��1��A��������______��B�����������ŵ�������______��
��2����Ӧ�ݵĻ�ѧ����ʽΪ______���÷�Ӧ�ķ�Ӧ������______��
��3��G�ķ���ʽΪ______��
��4��д����������������E��ͬ���칹��Ľṹ��ʽ��______��______��
������ֻ������ȡ����
��˴Ź�������ͼ��ֻ��4�����շ�
��.1mol������������NaHCO3��Һ��Ӧ����2molCO2
��5����������֪ʶ����������Ϣ��д����![]() Ϊԭ���Ʊ�
Ϊԭ���Ʊ�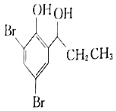 �ĺϳ�·������ͼ�����Լ���ѡ��______���ϳ�·������ͼʾ�����£�CH3CH2Br
�ĺϳ�·������ͼ�����Լ���ѡ��______���ϳ�·������ͼʾ�����£�CH3CH2Br![]() CH3CH2OH
CH3CH2OH![]() CH3COOCH2CH3
CH3COOCH2CH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�ҷ��ֶ�ұ�����е�⾫���ᴿ�ɽ��ߴ����Ʊ��ɱ�����ص���װ������ͼ����Cu��Si�Ͻ�����Դ����950����������Һ���ν��е�⾫����������ijCH4ȼ�ϵ�أ���ͼ����Ϊ��Դ���й�˵������ȷ����

A. �缫c��a������d��b����
B. �������У�Si ������Cu����ԭ��Cu������Si������
C. ����Һ���ε������������ⷴӦ�������߹����Ч��
D. ��ͬʱ���£�ͨ��CH4��O2�������ͬ����Ӱ����ᴿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�ǵ���������������������Ԫ��֮һ����Ҫ��ش�
��1��̼ԭ�Ӻ�����________�ֲ�ͬ�ռ��˶�״̬�ĵ��ӣ���һ�����ܽ���B��C֮���Ԫ�ص�����Ϊ_________��
��2��̼Ԫ�����γɶ������
��CO32-�����幹����_______________��
��MgCO3�ֽ��¶ȱ�CaCO3�͵�ԭ����_________________________��
��ʯī��ؿ��γ�ʯī�в����Ӿ���C8K����ͼ������ṹΪÿ��һ��̼ԭ�Ӳ���һ���ԭ�ӣ����ԭ�Ӳ����ڵ���������̼ԭ�����з�ʽ��ͬ�����������Ⱦ����λ̼ԭ����_________����

��3��̼Ҳ���γɶ����л��������ͼ��ʾ��һ�����ʺ�һ����वĽṹ�����ַ���������ԭ�Ӷ���һ��ƽ���ϡ�

��1 mol ��ष����к���������Ŀ��__________��
�����ʽṹ��Nԭ�ӵ��ӻ���ʽΪ________��
�������й��֮��ļнǡ�1�ȡ�2����ԭ��________________________________��
��4�����������ʯ�е�ÿ��̼ԭ����һ����4��̼ԭ����ɵ���������ṹ��Ԫȡ�����γ�̼��һ��������ά��������ṹ����T-̼����֪T-̼�ܶ�Ϊ�� g/cm�������ӵ�����ΪNA����T-̼�ľ�������a=________ pm (д������ʽ����)��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����͵��Ļ������ڹ������衢��ũҵ�����������ж��м���㷺����;����ش������뵪Ԫ���йص����⣺
(1)NaCN�㷺���ڵ�ƹ�ҵ�ϣ�ʵ����Ũ����ͬ��NaCN��Һ��NaHCO3��Һ��ǰ�ߵ�pH�������ԣ�HCN___________H2CO3(����ǿ��������������)��
(2)��������(�ṹʽΪCl��N=O)���л��ϳ��е���Ҫ�Լ���������C12��NO��ͨ�������·�Ӧ�Ƶã���Ӧ����ʽΪ��2NO(g)+Cl2(g)![]() 2ClNO(g)����֪���ֻ�ѧ���ļ����������±���
2ClNO(g)����֪���ֻ�ѧ���ļ����������±���

��Cl2��NO��Ӧ����ClNO�Ĺ�����ת����5mol���ӣ������������仯Ϊ___________kJ��
(3)��һ�������ܱ������г���2 mol NO(g)��1molCl2(g)����(2)�з�Ӧ�����¶ȷֱ�ΪT1��T2ʱ���NO�����ʵ���(��λ��mol)��ʱ��Ĺ�ϵ���±���ʾ��

��T1___________T2(����>�����ܡ�����=��)��������___________��
���������ݻ�Ϊ1L���¶�ΪT1��ʱ����Ӧ��ʼ��5minʱ��C12��ƽ����Ӧ����Ϊ_______��
���¶�ΪT2��ʱ������ͬ�����У�����1 molNO(g)��0.5mo1Cl2(g)����NO��ƽ��ת����___________50%(������������������������С����)
���¶�ΪT2��ʱ����ʼʱ�����ڵ�ѹǿΪp0����÷�Ӧ��ƽ�ⳣ��Kp=___________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
(4)������������ˮ�еĵ���Ⱦ�ѳ�Ϊһ�������ԵĻ������⡣�ڽ���Pt��Cu��ҿ(Ir)�Ĵ������£��ܱ������е�H2�ɸ�Чת��������Һ�е���̬��(NO3��)���乤��ԭ����ͼ��ʾ��

��Ir���淢����Ӧ�ķ���ʽΪ___________��
������������ϵ�Pt�������࣬��ɵĽ����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״����11.2LCH4�ڿ�������ȫȼ�գ�������������⣺
(1)д���÷�Ӧ�Ļ�ѧ����ʽ_______��
(2)�����״����11.2LCH4������_______��
(3)��������CO2������_______��[(2)(3)��������]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ�����ӵ�������ֵ������˵����ȷ����
A. 1molH2��1molI2�ڼ��������³�ַ�Ӧ������HI�ķ�����Ϊ2NA
B. 10g��������Ϊ46%���Ҵ���Һ���е���ԭ����ĿΪ0.6NA
C. 20mL0.1 mol/LAlCl3��Һ�У�ˮ���γ�Al(OH)3����������Ϊ0.002NA
D. 0.1molNa2O2��Na2O�Ļ�����к��е�������������0.3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й���ʵ�������˵����ȷ���ǣ� ��
A. �ñ���ȡ��ˮ�е���ʱ������ı���Һ�ӷ�Һ©���¿ڷų�
B. �к͵ζ�ʵ���У���ƿ������ˮϴ���뾭�����ɺ�ſ�ʹ��
C. ����0.5mol��L��1480mL��NaOH��Һ�����9.6 g NaOH����
D. ij��Һ�е���2��K3[Fe(CN)6]��Һ���ɾ���������ɫ�ij�����˵��ԭ��Һ�к���Fe2+
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com