
����Ŀ��ijʵ��С��������װ�ý����Ҵ���������ʵ�顣
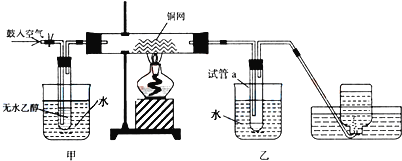
��1��ʵ�������ͭ�����ֺ�ɫ�ͺ�ɫ�����������д����Ӧ�Ļ�ѧ��Ӧ����ʽ��___��__��
��2���ڲ��Ϲ������������£�Ϩ��ƾ��ƣ���Ӧ���ܼ������У�˵�����Ҵ�������Ӧ��__��Ӧ��
��3����������ˮԡ���ò���ͬ����������__���ҵ�������__��
��4����Ӧ����һ��ʱ����Թ�a�����ռ�����ͬ�����ʣ�������__������ƿ���ռ������������Ҫ�ɷ���___����д���ƣ�
��5�����Թ�a���ռ�����Һ����ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ���л�����__����ȥ�����ʣ������ڻ��Һ�м���___(��д��ĸ)��
a.�Ȼ�����Һ b.�� c.̼��������Һ d.���Ȼ�̼
Ȼ����ͨ��___(��ʵ���������)���ɳ�ȥ��
���𰸡�2Cu+O2![]() 2CuO CH3CH2OH+CuO
2CuO CH3CH2OH+CuO![]() CH3CHO+Cu+H2O ���� �����Ҵ��������Ҵ��Ļӷ� ��ȴ��������ȩ���ռ� ��ȩ���Ҵ���ˮ ���� ���� C ����
CH3CHO+Cu+H2O ���� �����Ҵ��������Ҵ��Ļӷ� ��ȴ��������ȩ���ռ� ��ȩ���Ҵ���ˮ ���� ���� C ����
��������
��1���Ҵ��Ĵ�����ʵ����Cu��O2��Ӧ���ɺ�ɫCuO��CuO���Ҵ���Ӧ����Cu����ȩ��������Cu��O2��Ӧ�������ֺ�ڽ�������
��2���������ܷ�Ӧ��˵���÷�Ӧ���ȣ�
��3�����в����Ҵ����������з�Ӧ����Ӧ�����ɵ���ȩ������������
��4�����Ⱥ�δ��Ӧ�ķ�Ӧ���Ҵ���O2�������Ҵ��������б�������
��5�����Թ�a���ռ�����Һ����ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ�������ᣬֻ����Ϊ���ᣬ�ݴ˻ش�
��1���ɷ�����֪��ͭ�����ֺ�ڽ��������������������Ӧ��2Cu+O2![]() 2CuO ��CH3CH2OH+CuO
2CuO ��CH3CH2OH+CuO![]() CH3CHO+Cu+H2O���ʴ�Ϊ��2Cu+O2
CH3CHO+Cu+H2O���ʴ�Ϊ��2Cu+O2![]() 2CuO��CH3CH2OH+CuO
2CuO��CH3CH2OH+CuO![]() CH3CHO+Cu+H2O��
CH3CHO+Cu+H2O��
��2���������ܷ�Ӧ��˵���÷�Ӧ���ȣ���Ϊ�����ȣ�
��3����������ʹ�Ҵ��ӷ��ģ�ӦΪ��ˮԡ��������ʹ��ȩ�����ģ�ӦΪ��ˮԡ���ˮԡ���ʴ�Ϊ�������Ҵ��������Ҵ��Ļӷ�����ȴ��������ȩ���ռ���
��4�����Ⱥ�δ��Ӧ�ķ�Ӧ���Ҵ���O2�������Ҵ��������б�����������ƿ���ռ��������뷴Ӧ�ĵ������ʴ�Ϊ����ȩ���Ҵ���ˮ��������
��5�����Թ�a���ռ�����Һ����ɫʯ����ֽ���飬��ֽ�Ժ�ɫ��˵��Һ�������ᣬ��������һ������ȩ�����������ᣬ���ȥ���ᣬ����NaHCO3���䷴Ӧ��Ȼ���������ᴿ���ʴ�Ϊ�����C������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
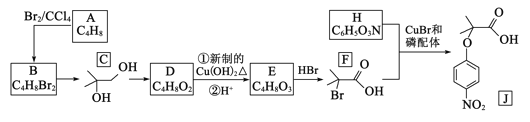
����Ŀ���л���J���ڴ�λ����ϵ���е�һ������,���л������������ʮ����Ҫ�ļ�ֵ.2018���ҹ��״�ʹ����������ʻ�������ϳɴ�λ����J,��ϳ�·������:
��֪��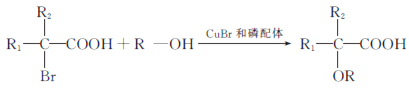
�ش���������:
(1)A ������___________________.
(2)C ��D�Ļ�ѧ����ʽ_________________________.E ��F�ķ�Ӧ����____
(3)H �к��еĹ�����________________.J�ķ���ʽ_______________.
(4)������X��D��ͬ���칹��,������������������Һ��Ӧ��X��_____________��(�����������칹),д�����к˴Ź���������3���,�����֮��Ϊ1��1��6�Ľṹ��ʽΪ___________________________.
(5)�������кϳ�·��ͼ���漰�Լױ���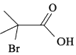 Ϊԭ�����ϳ���һ�ִ�λ����
Ϊԭ�����ϳ���һ�ִ�λ����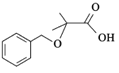 �ĺϳ�·�ߣ�__________________��
�ĺϳ�·�ߣ�__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���У��ܴﵽ��Ӧʵ��Ŀ�ĵ���
|
|
|
|
A���Ʊ����ռ��������� | B��֤���Ȼ����ܽ�ȴ������� | C����֤���������ȥ��������ϩ | D���ƶ�S��C��Si�ķǽ�����ǿ�� |
A.AB.BC.CD.D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ��������ʣ���������δ���������ϩ![]() A
A![]() �Ҵ�
�Ҵ�![]() ��ȩ
��ȩ![]() ���ᡣ������Ҫ��д����
���ᡣ������Ҫ��д����
(1)�Ҵ��к�������������Ϊ________�������к�������������Ϊ________
(2)д�����з�Ӧ����ʽ��
��Ӧ��____________
��Ӧ�ܣ�____________
(3)��Ӧ�ܵ�ʵ������Ϊ��___________��ʵ���ҳ���������Һ������ȩ�������õ�ʵ�Լ�2%��������Һ��2%ϡ��ˮ��������Һ���Ƶķ����ǣ�______
(4)��ӦΪ�Ҵ��������ڼ��ȼ�Cu�������·�Ӧ������ȩ������д����Ӧ��ͭ˿�ɺ��ڣ����ɺڱ������еĻ�ѧ����ʽ��__________��_______��
(5)������ͼ��ʾװ���Թ�a���ռ��Ҵ��������IJ��
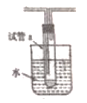
��ˮԡ������Ϊ_______________������ɫʯ����ֽ�����ռ��IJ����ֽ�Ժ�ɫ��˵��Һ���л�����__________��
��Ҫ��ȥ�����ʣ����ڻ��Һ�м���______����д��ĸ��
a.�Ȼ�����Һ b.�� c.̼��������Һ d.���Ȼ�̼
Ȼ��,��ͨ��________����ʵ��������ƣ����ɳ�ȥ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ���������ƿ�����ýȾ������ʴ���ȡ�ʵ������ͭ��Ũ���ᡢ��������Ϊԭ���Ʊ��������Ƶ�װ����ͼ��ʾ�����ּг�װ���ԣ�����֪��NO��NO2���ܱ�����KMnO4��Һ����ΪNO3-��
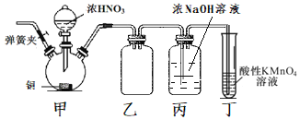
��1������װ�ú�����Ҫ���еIJ�����___��Ȼ��װ��ҩƷ��
��2��װ�ü��з�Ӧ�����ӷ���ʽΪ___��
��3��װ���ҵ�������___��
��4��ʵ�������װ�õĵ��ɼУ�ͨ��N2����Ŀ����___��
��5��װ�ö���������___��
��6��Ϊ�˲ⶨ����NaNO2��Ũ�ȣ�ȡ������Һ20.00mL����0.1000mol/L������KMnO4��Һ���еζ�������KMnO4��Һ10.00mL����ش�
������KMnO4��Һʢ����___�ζ����С�
���жϵζ����յ�ʱ������Ϊ___��
��д��������Ӧ�����ӷ���ʽ___�������NaNO2��Ũ��Ϊ___mol/L��
����������KMnO4��Һ����ʱ����������ƿ�Ŀ̶��ߣ�NaNO2��Ũ��___�����ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������������þ�Ļ����Һ�У���μ���ϡ����������Һ��ֱ�����������б�ʾ�������Ƽ�����(X)����Һ�г��������(Y)�Ĺ�ϵʾ��ͼ����ȷ���ǣ� ��

A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ȣ�ClO2���㷺Ӧ����ֽ��Ư�ס�ɱ��������ˮ��������������ҵ�����ü״���ԭNaClO3�ķ����Ʊ�ClO2�������������£�
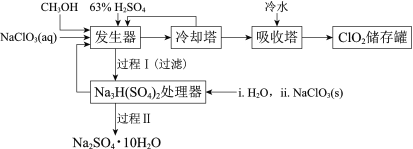
��֪��a�����������Ʊ�ClO2�ķ�Ӧ��12NaClO3+8H2SO4+3CH3OH= 12ClO2��+3HCOOH+4Na3H(SO4)2��+9H2O
b��������ʵ��۷е㣺
���� | CH3OH | HCOOH | ClO2 |
�۵�/�� | ��97 | 9 | ��59 |
�е�/�� | 65 | 101 | 11 |
(1)ClO2������ֽ��Ư�ס�ɱ���������������______�ԡ�
(2)��ȴ�����ڷ���ClO2������CH3OH��Ӧ���Ƶ�����¶�Ϊ______������ĸ����
A��0~10�� B��20~30�� C��60~70��
(3)�����̢���̢���Ի��â����Na2SO4��10H2O����ʹ����ԭ��ѭ�����á�
��֪��Na2SO4��10H2O��Na2SO4���ܽ����������ͼ��
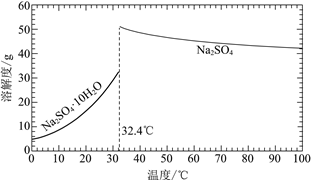
��Na3H(SO4)2�������л��â��ʱ�����NaClO3���壬��â���ܽ�ƽ��ĽǶȽ�����ԭ��______��
�ڽ��Na2SO4��10H2O��Na2SO4���ܽ�����ߣ����̢�IJ����ǣ���32.4�����������______��
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ����NaClO3��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ�����ڱ���һ���֣��ش������й����⣺
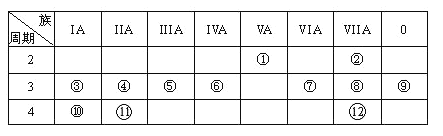
��1��д��Ԫ�آ�Ԫ�ط��ţ�__________��Ԫ�آ����ӵĵ���ʽ__________��
��2������Ԫ�آܵ�ԭ�ӽṹʾ��ͼ��________________________��
��3������ЩԪ���У�����õ�Ԫ����__________��дԪ�ط��ţ���
��4������ЩԪ�ص�����������Ӧˮ�����У�������ǿ����___________��������ǿ����_____________�������Ե�����������_______________�������Ͼ�Ҫ��д��ѧʽ��
��5���Ʋ�50��Ԫ�������ڱ��е�λ�ã�_________________________________��
��6��Ԫ�����ڱ��У�Ԫ���������������γɵĵ��ʺͻ���������ʾ��������Ա仯���ɣ���д��һ����������Ԫ�����γɵĻ�����ij�����ʵı仯���ɣ�__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)�л���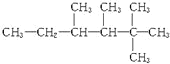 ��ϵͳ����������_________
��ϵͳ����������_________
(2)��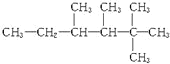 ��ij��ϩ���������ӳɺ�IJ�����ϩ��������___________�ֽṹ������ijȲ���������ӳɺ�IJ�����Ȳ��������___________�ֽṹ��
��ij��ϩ���������ӳɺ�IJ�����ϩ��������___________�ֽṹ������ijȲ���������ӳɺ�IJ�����Ȳ��������___________�ֽṹ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com