
����Ŀ���������ȣ�ClO2���㷺Ӧ����ֽ��Ư�ס�ɱ��������ˮ��������������ҵ�����ü״���ԭNaClO3�ķ����Ʊ�ClO2�������������£�
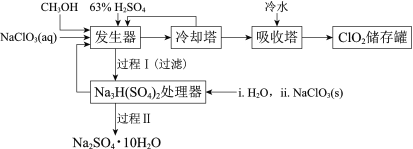
��֪��a�����������Ʊ�ClO2�ķ�Ӧ��12NaClO3+8H2SO4+3CH3OH= 12ClO2��+3HCOOH+4Na3H(SO4)2��+9H2O
b��������ʵ��۷е㣺
���� | CH3OH | HCOOH | ClO2 |
�۵�/�� | ��97 | 9 | ��59 |
�е�/�� | 65 | 101 | 11 |
(1)ClO2������ֽ��Ư�ס�ɱ���������������______�ԡ�
(2)��ȴ�����ڷ���ClO2������CH3OH��Ӧ���Ƶ�����¶�Ϊ______������ĸ����
A��0~10�� B��20~30�� C��60~70��
(3)�����̢���̢���Ի��â����Na2SO4��10H2O����ʹ����ԭ��ѭ�����á�
��֪��Na2SO4��10H2O��Na2SO4���ܽ����������ͼ��
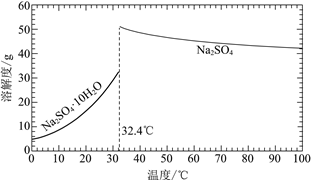
��Na3H(SO4)2�������л��â��ʱ�����NaClO3���壬��â���ܽ�ƽ��ĽǶȽ�����ԭ��______��
�ڽ��Na2SO4��10H2O��Na2SO4���ܽ�����ߣ����̢�IJ����ǣ���32.4�����������______��
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ����NaClO3��______��
���𰸡����� B ![]() ������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O������ ��ȴ�ᾧ�����ˣ�ϴ�ӣ����� H2SO4
������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O������ ��ȴ�ᾧ�����ˣ�ϴ�ӣ����� H2SO4
��������
�����̿�֪�����������Ʊ�ClO2, ��ȴ�����ڷ���ClO2������CH3OH����������������ˮ����ClO2������ٴ��棻�����������ɵ�Na3H(SO4)2����Na3H(SO4)2����������H2O2��NaClO3�����õ�Na2SO4��10H2O��
��1��ClO2������ֽ��Ư�ס�ɱ��������������������ԣ��ʴ�Ϊ��������
��2����ȴ�����ڷ���ClO2������CH3OH�����ݱ�����������ʵ��ܽ�ȣ����Ƶ��¶�Ӧ��ʹCH3OHҺ��������ClO2����Һ����ֻ��B����ʣ���ѡB��
��3�����ڴ������лᷢ����Ӧ��![]() ������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O���������ʴ�Ϊ��
������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O���������ʴ�Ϊ��![]() ������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O��������
������NaClO3��ʹ������Ũ������ƽ�������ƶ���������Na2SO4��10H2O��������
�ڹ��̢�IJ���Ϊ��32.4���������������ȴ�ᾧ�����ˣ�ϴ�ӣ�����ʴ�Ϊ����ȴ�ᾧ�����ˣ�ϴ�ӣ����
��Na3H(SO4)2����������Һ�п���ѭ�����õ�ԭ��ΪNaClO3��H2SO4,�ʴ�Ϊ��H2SO4��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ�ǽ��ʯ�ľ����ṹ�����������������̼ԭ���⣬4����Խ��ߵ�1/4��������1��̼ԭ�ӡ��ش��������⣺

(1)��ͼ��ԭ��1������Ϊ![]() ����ԭ��2������Ϊ________�������ʯ�ľ�������Ϊ
����ԭ��2������Ϊ________�������ʯ�ľ�������Ϊ![]() ��������̼̼���ļ���
��������̼̼���ļ���![]() ________pm(�ú�
________pm(�ú�![]() �Ĵ���ʽ��ʾ)��
�Ĵ���ʽ��ʾ)��
(2)��������![]() ��������ʯ�ṹ���ƣ����������Ӹ�ռ��������������һ�롣
��������ʯ�ṹ���ƣ����������Ӹ�ռ��������������һ�롣
��SԪ�ؼ���ͬ���ڵ�����Ԫ�ص�һ��������С�����˳����________�����Ȼ���(![]() )������Sԭ�ӵ��ӻ�������________��
)������Sԭ�ӵ��ӻ�������________��
��д����̬![]() �ĵ����Ų�ʽ________���Ѿ���ʾ��ͼ�б�ʾ
�ĵ����Ų�ʽ________���Ѿ���ʾ��ͼ�б�ʾ![]() ��С��ȫ��Ϳ��_______��
��С��ȫ��Ϳ��_______��
��п�̸ɵ����![]() �����յ�ط�Ӧ������
�����յ�ط�Ӧ������![]() ����
����![]() �����������________��
�����������________��![]() ����
����
������![]() �����ľ�������Ϊ
�����ľ�������Ϊ![]() �������ӵ�������ֵΪ
�������ӵ�������ֵΪ![]() ��������ܶ�Ϊ________
��������ܶ�Ϊ________![]() (�г�����ʽ)
(�г�����ʽ)
(3)����������ľ���ҲӦ�������ڽ��ʯ����ʵ�ʽ�Ϊ���ӣ���������Ϊ��������������������Ժͷ����������ѱ��ϸ�ִ�С����������ױ�����������ÿ����ԭ�Ӷ��γ��������ÿ��![]() ����Χ________��
����Χ________��![]() ͨ��������ϡ�
ͨ��������ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ѳ�����ɫ��������Ӳ��ǿ�ȴ����ȡ��ܶ�С������Ϊ����������Ŀǰ�����Ѳ����Ȼ������������ʯ���������뽹̿��ϣ�ͨ�������������Ƶ�TiCl4��2FeTiO3+7Cl2+6C![]() 2TiCl4+2FeCl3+6CO�� TiO2+2Cl2+2C
2TiCl4+2FeCl3+6CO�� TiO2+2Cl2+2C![]() TiCl4+2CO����TiCl4�����ᴿ���������������þ���ȵõ��ѣ�TiCl4+2Mg
TiCl4+2CO����TiCl4�����ᴿ���������������þ���ȵõ��ѣ�TiCl4+2Mg![]() Ti+2MgCl2��MgCl2����Mg��ϡ�����ܽ��ú���״�ѣ���������ۻ������Ѷ�����ش��������⣺
Ti+2MgCl2��MgCl2����Mg��ϡ�����ܽ��ú���״�ѣ���������ۻ������Ѷ�����ش��������⣺
��1����̬��ԭ�ӵļ۵����Ų�ʽΪ_______________________________��
��2����CO��Ϊ�ȵ����������Ϊ_____���ѧʽ����
��3����CH2Cl2��C6H6��CO2��C2H4�У�̼ԭ�Ӳ�ȡsp�ӻ��ķ�����__________ ��
��4��TiCl4�ڳ���������ɫҺ�壬��ˮ��ʪ��������ˮ���ð���̡���TiCl4����______(�ԭ�ӡ��������ӡ������ӡ�)���塣
��5������ͬ���ڵ���һ��Ԫ����(Co)���γɷ���ʽ��ΪCo(NH3)5BrSO4��������������һ�ֻ�ѧʽΪ[Co(NH3)5Br]SO4��������Һ�м�BaCl2��Һʱ��������_____________������һ����������Һ�м���BaCl2��Һʱ������������������AgNO3��Һʱ����������ɫ��������ڶ��������Ļ�ѧʽΪ __________��
��6������Ȼ����TiO2�н��ʯ�����ѿ����ѿ����־��ͣ����н��ʯ�ľ�������ͼ��ʾ��������Ti4+����λ��Ϊ__________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ��������������ȷ����
A. l00g 9%��������ˮ��Һ����ԭ����Ϊ0.3 NA
B. ��״���£�2.24L F2ͨ����������ʳ��ˮ�п��û���0.1NA��Cl2
C. ��ҵ�ϳɰ�ÿ����NA��N��N����ͬʱ����6NA��N-H������Ӧ�ﵽƽ��
D. ������l LpH=7��1mol/LCH3COONH4��Һ��CH3COO-��NH4+��Ŀ��ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��HI�������л���Ӧ�еĻ�ԭ�������Ȼᷢ���ֽⷴӦ����֪![]() ʱ��
ʱ��![]() ����1L�ܱ������г���1molHI��
����1L�ܱ������г���1molHI��![]() ʱ����ϵ��
ʱ����ϵ��![]() �뷴Ӧʱ��t�Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ����
�뷴Ӧʱ��t�Ĺ�ϵ��ͼ��ʾ������˵���У���ȷ����

A.![]() min�ڵ�ƽ����Ӧ���ʿɱ�ʾΪ
min�ڵ�ƽ����Ӧ���ʿɱ�ʾΪ![]()
![]()
B.�����¶ȣ��ٴ�ƽ��ʱ��![]()
![]()
C.�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ![]()
D.��Ӧ����40minʱ����ϵ���յ�����ԼΪ![]() kJ
kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾΪ�����������ṹ��ͼ�������ཻ����A��B��C��D ��ʾ���ʼ�ķ�Ӧ�����ж�Ӧ���ַ�Ӧ�����ӷ���ʽ��д����ȷ����
![]()
A��Cl2��2OH��=Cl����ClO����H2O
B��Cu2����2OH��=Cu(OH)2��
C��Cu2����SO42-��Ba2����2OH��=BaSO4����Cu(OH)2��
D��OH����HCO3-=H2O��CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾ�������������ǣ��� ����

A. Ba2����Mg2����NO3-��CO32-B. H����Ba2����Al3����Cl��
C. K����Ba2����Cl����HCO3-D. NH4+��Ba2����Fe2����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԫ��X��Y��Z��W��ԭ��������������������Ԫ����ɵĵ��ʻ���A��B��C��D��E��F��G֮����ת����ϵ��ͼ��ʾ(��Ӧ��������ȥ)�����н�BΪ���ʣ�DΪ����ɫ���壬A�㷺�����������С�����˵������ȷ���ǣ� ��

A.�����Ӱ뾶��Z>W
B.���⻯����ȶ��ԣ�Z>Y
C.D��F��G�������Ļ�ѧ��������ȫ��ͬ
D.1molD�ֱ�������C��E��Ӧʱ��ת�Ƶĵ�����Ŀ��ΪNA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������ۻ������Ӽ�˷���__�������������������ۻ������Ӽ�˷���__����������������������Ӽ�˷���__�������������־�����۵��ɸߵ��͵�˳����__��
��2���������־��壺��CO2����NaCl����Na����Si����CS2�����ʯ�����ǵ��۵�ӵ͵��ߵ�˳��Ϊ__������ţ���
��3����H2����NH4��2SO4��SiC��CO2��HF�У��ɼ��Լ��γɵķǼ��Է�����__���ɷǼ��Լ��γɵķǼ��Է�����__�����γɷ��Ӿ����������__����������ľ���Ļ�ѧʽ��__���������Ӿ������__������ԭ�Ӿ������__���������ʵ��۵��ɸߵ��͵�˳����__��
��4��A��B��C��DΪ���־��壬�������£�
A����̬ʱ�ܵ��磬����������
B��������CS2��������ˮ
C����̬ʱ�����磬Һ̬ʱ�ܵ��磬������ˮ
D����̬��Һ̬ʱ�������磬�۵�Ϊ3 500��
���ƶ����ǵľ������ͣ�A��__��B��__��C��__��D��__��
��5��ͼ��A��D����ѧ��ѧ�̿����ϳ����ļ��־���ṹģ�ͣ�����д��Ӧ���ʵ����ƣ�
A��__��B��__��C��__��D��__��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com