
����Ŀ��ijŨ����������ǩ��ͼ����ͼ��ʾ�����ø�Ũ��������250mLŨ��Ϊ1mol/L��ϡ���ᡣ�Իش��������⣺

��1����Ũ�����Ũ��Ϊ_____________��
��2�������㣬��ѡ��������______������ĸ������Ͳ����ȡ_____�����������mLŨ���ᡣ
A. 5 mL B. 10 mL C. 25 mL D. 50 mL
��3������ȡŨ������������в�����
�ٵ�ϡ�ͺ�������¶�������һ�º��ز�����������ע������ƿ�С�
��������ƿ��С�ļ�����ˮ��Һ��������ƿ���̶�����1��2 cm�������ý�ͷ�ιܼ�����ˮ��ʹ��Һ�İ�Һ����ʹ���̶������С�
����ʢŨ������ձ���ע������ˮ�����ò��������裬ʹ���Ͼ��ȡ�
��������ˮϴ���ձ��Ͳ�����2��3�Σ�����ϴ��Һȫ��ע������ƿ�С�
���������У���ȷ��˳���ǣ�����ţ�___________________��
��4����ʵ��������Һ�Ĺ����У��������ڲ�������������������������ҺŨ�Ȳ���Ӱ�죬�������������Ա�ʵ��������ϡ����Ũ�ȵ�Ӱ�죨����ƫ��������ƫ����������Ӱ��������
�ٶ���ʱ���ӿ̶��ߣ��ᵼ������ϡ����Ũ��_______��
���øո�ϴ�Ӹɾ�����Ͳ����ȡŨ���ᣬ�ᵼ������ϡ����Ũ��__________��
���𰸡�12mol/L C 20.8mL �ۢ٢ܢ� ƫ�� ƫ��
��������
(1)������![]() ����Ũ��������ʵ���Ũ�ȣ�
����Ũ��������ʵ���Ũ�ȣ�
(2)����Һϡ�������������ʵ����ʵ������ֲ��䣬�ݴ˼�����ҪŨ�������������Ũ�������ѡ����Ͳ���
(3)����������һ�����ʵ���Ũ����Һ��һ�㲽������
(4)����![]() ���
���
(1)����������Ϊ36.5%��Ũ����(�ܶ�Ϊ1.2g/cm3)�����ʵ���Ũ��![]() ��
��
�ʴ�Ϊ��12mol/L��
(2)�����Ƴ�1mol/L��ϡ����250ml��Ӧѡ��250mL����ƿ��ʵ������250mL��Һ������ҪŨ�������ΪV����Һϡ�������������ʵ����ʵ������ֲ����֪��V��12mol/L= 1.0mol/L��250mL�����V=20.8mL��Ӧѡ��25mL��Ͳ��
�ʴ�Ϊ:��C ��20.8mL��
(3)������һ�����ʵ���Ũ����Һ��һ�㲽��:���㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ�������ȷ��˳��Ϊ���ۢ٢ܢڣ�
�ʴ�Ϊ���ۢ٢ܢڣ�
(4)�١�����ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫС������![]() ��֪����ҺŨ��ƫ�ߣ�
��֪����ҺŨ��ƫ�ߣ�
�ڡ��øո�ϴ�ӽྻ����Ͳ����ȡŨ���ᵼ��Ũ���ᱻϡ�ͣ���ȡ���������Ȼ�������ʵ���ƫС,����![]() ��֪����ҺŨ��ƫ�ͣ�
��֪����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ߣ�ƫ�͡�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����10mL0.1mol��L-1 NH4Al(SO4)2��Һ��,�μӵ�Ũ��Ba(OH)2��ҺxmL������������ȷ���ǣ� ��
A. x=10ʱ����Һ����NH4+��Al3+��SO42-����c(NH4+)��c(SO42-)
B. x=10ʱ����Һ����NH4+��AlO2-��SO42-����c(NH4+)��c(Al3+)
C. x=30ʱ����Һ����Ba2+��AlO2-��OH-����c(OH-)��c(Ba2+)
D. x=30ʱ����Һ����Ba2+��AlO2-��OH-����c(OH-)��c(AlO2-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���£������ʢ�44.8LH2����24gCH4����1molH2O����3.01��1023��O2����������������______������ţ���ͬ������������������______������������______�������С����______���ܶ���С�����˳��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ͻ���и�Ӳ�ȡ���ʴ���ԣ��㷺Ӧ���ھ����������졣�ɸ�̼�����Ͻ���Ϸ�ĩ��ȡ���ļ��������£�
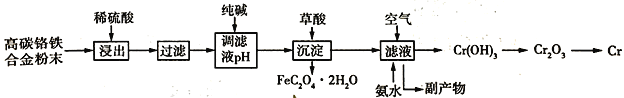
��֪��Cr + H2SO4 = CrSO4 + H2��
��ش��������⣺
��1��ϡ������������У���������������Ĵ�ʩ��______________��������һ�����ɣ�
��2���ô��������Һ��ȣ����������������ܵ��µĺ����_________________��������ֱ���ŷŻ��������˷ѣ�����Ⱦˮ�ʡ�����д���������һ����;______________���������֣���
��3��������ᣨH2C2O4�����������ӷ���ʽΪ__________________�����������۽�����д����ҵ��ұ�����Ļ�ѧ����ʽ_________________��
��4������Һ��ͨ����������백ˮ������Ӧ�Ļ�ѧ����ʽΪ_________________��
��5����֪��̼��������������Ԫ������֮��Ϊ14:13��������������Ԫ��ת��Ϊ����������������Ϊ80%����������ȡ����������ת����Ϊ95%������õ������������壨FeC2O42H2O��������Ϊ18.00 t �������ұ����������Ϊ________t���������1λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�������Ʊ����ȱ�����ķ�Ӧ�Լ�װ����ͼ��ʾ��
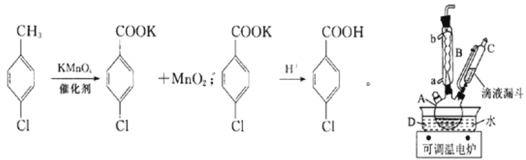
�����£����ʵ��й����ݺ����������ʾ��
�۵�/�� | �е�/�� | �ܶ�/g��cm��3 | ��ɫ | ˮ���� | |
���ȼױ� | 7.5 | 162 | 1.07 | ��ɫ | ���� |
���ȱ����� | 243 | 275 | 1.54 | ��ɫ | �� |
���ȱ������ | �����ε�ͨ�ԣ����ڿ������� | ||||
ʵ�鲽�裺�ڹ��Ϊ250mL������A�м���һ�����Ĵ���������KMnO4��100mLˮ����װ��װ�ã��ڵ�Һ©���м���6.00mL���ȼױ������¶�Ϊ93������ʱ����ε�����ȼױ��������¶���93�����ң���Ӧ2h�����ˣ�����������ˮϴ�ӣ�ʹϴ��Һ����Һ�ϲ�������ϡ�����ữ������Ũ������ȴ��Ȼ����ˣ�����������ˮ����ϴ�ӣ�����������������
��ش��������⣺
(1)����A������Ϊ______________________��
(2)����B�������ܣ�������Ҫ�����ǣ�________________��ʵ������У���ȴˮ��________�ڡ�
(3)ʵ����������ι��ˡ�ϴ�Ӳ�������һ�ι��˵������ɷ�Ϊ___________(�ѧʽ)��ϴ�Ӹ���������ˮ��Ŀ����_________________________________���ڶ��ι��˺�ϴ����������ˮ��Ŀ����______________________��
(4)���ˡ�ϴ�Ӳ��������õ���������___________(��ѡ����ĸ)��
a.�ձ� b.��Һ©�� c.��ƿ d.������
(5)��һ�ι��˺����Һ�м������ᣬ���ֵ�������___________��
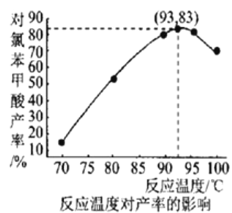
(6)��ͼ���¶ȶԶ��ȱ�������ʵ�Ӱ���ϵ������������õ��Ķ��ȱ����������Ϊ___________(����С�������λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ������ͼ��ʾʵ��װ�ý�������ˮ������Ӧ��ʵ�飬���Է�Ӧ��Ӳ���Թ��й������ʵ���ɽ�����̽����
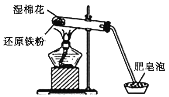
��ش��������⣺
��1��Ӳ���Թ���ʪ����������________________��
��2������ˮ�����ķ�Ӧ�У���������________���ѧʽ����ͬ������ԭ����________��
��3����ͬѧ�Է�Ӧ��Ӳ���Թ��й������ʵ������������¼��裺
����1��ֻ��Fe��
����2��ֻ��________��
����3������FeҲ��Fe3O4��
��4��Ϊ����֤��Ӧ��Ӳ���Թ��й������ʵ���ɣ���ͬѧ�����˶����о�����Ӧǰ�������ʵ�����Ϊ5.6 g����Ӧ��������ʵ�����Ϊ6.6 g�����������ݷ�������Ӧ��Ӳ���Թ��й������ʵ����Ϊ__________________��д���÷�Ӧ�Ļ�ѧ����ʽ________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ���ҿ���KMnO4��Ũ���ᷴӦ��ȡ������
KMnO4��HCl��Ũ��=KCl��MnCl2��Cl2����H2O��δ��ƽ��
��1����ƽ��ѧ����ʽ�����õ����ű������ת�Ƶķ������Ŀ��
��2����������ƽ�Ļ�ѧ����ʽ��дΪ���ӷ���ʽ ��
��3��Ũ�����ڷ�Ӧ����ʾ������������________________
��ֻ�л�ԭ�� ����ԭ�Ժ�����
��ֻ�������� �������Ժ�����
��4��������0.5molCl2����������HCl mol��ת�Ƶĵ��ӵ���ĿԼΪ___________��
��5��һ�������£�KMnO4����������������ԭ�����ʡ�
MnO4��+C2O42��+ = Mn2+ +CO2��+
����������ӷ���ʽ���˷�Ӧ�У�����������Ӧ�������� ����ת��1mol���ӣ����ɱ�״����CO2 L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijһ��Ӧ��ϵ���з�Ӧ��������ﹲ�������ʣ�O2��H2CrO4��Cr (OH)3��H2O��H2O2�� ��֪�÷�Ӧ��H2O2ֻ�������¹��̣�H2O2��O2
(1)�÷�Ӧ�еĻ�ԭ����______________��
(2)�÷�Ӧ�У�������ԭ��Ӧ�Ĺ�����________��________��
(3)д���÷�Ӧ�Ļ�ѧ����ʽ�����õ����ű������ת�Ƶķ������Ŀ��__________��
(4)�練Ӧת����0.3 mol���ӣ�������������ڱ�״�������Ϊ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������![]() ������������ͨ������·�ߺϳɣ�
������������ͨ������·�ߺϳɣ�

��1������ȡ����Ӧ����______�����ڼӳɷ�Ӧ����______������ţ���
��2��A�Ľṹ��ʽΪ______��
��3��д����Ӧ�ܡ��ߵĻ�ѧ����ʽ��______��______��
��4������ʽΪC4H8BrCl���л��ﹲ��________�֡�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com