
����Ŀ����ȥұ��ҵ�ŷ�������![]() �ķ����ж��֡�
�ķ����ж��֡�
��1�����ñ�����Bunsen���Ȼ�ѧѭ������![]() ����������������Ӧ��ɣ�
����������������Ӧ��ɣ�
![]()
![]()
![]()
![]()
![]()
![]()
��![]()
![]() ________
________![]() ��
��
��2������п���շ�������![]() ����Һ�����������з��ѭ�������pH������Ч��
����Һ�����������з��ѭ�������pH������Ч��![]() ��ʱ��t�ı仯��ͼ�ף���Һ�в�������pH�Ĺ�ϵ��ͼ����ʾ��
��ʱ��t�ı仯��ͼ�ף���Һ�в�������pH�Ĺ�ϵ��ͼ����ʾ��
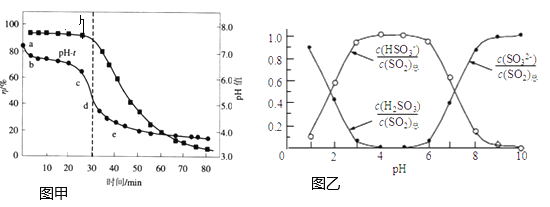
��Ϊ���![]() ������Ч��
������Ч��![]() ���ɲ�ȡ�Ĵ�ʩ�У���������Һ��
���ɲ�ȡ�Ĵ�ʩ�У���������Һ��![]() ������________��
������________��
��ͼ���е�![]() ����ab�η�������Ҫ��ѧ����ʽΪ________��
����ab�η�������Ҫ��ѧ����ʽΪ________��
��![]() ʱ����Һ
ʱ����Һ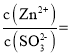 ________��
________��
��3����ͼ����ʾ�����ö��Ե缫��⺬![]() ����������S��
����������S��![]() ����ʵ�ַ��������á�
����ʵ�ַ��������á�
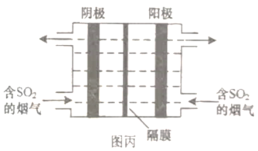
�������ĵ缫��ӦʽΪ________��
��ÿ������![]() �������������ϻ���S��
�������������ϻ���S��![]() �����ʵ����ֱ�Ϊ________��________��
�����ʵ����ֱ�Ϊ________��________��
���𰸡�![]() ������Һ��pH��6.8����
������Һ��pH��6.8���� ![]()
![]()
![]() 0.1mol 0.2mol
0.1mol 0.2mol
��������
��1����֪����2H2(g)+O2(g)=2H2O(l) H1=-572kJ��mol-1
��2HI(g)=H2(g)+I2(g) H2=+10kJ��mol-1
��2H2SO4(l)=2SO2(g)+2H2O(g)+O2(g) H3=+462kJ��mol-1
����ݸ�˹���ɿ�֪-(����2+��+����2)���õ�SO2(g)+I2(g)+2H2O(l)=2HI(g)+H2SO4(l) H=+45kJ��mol-1��
��2������������Һ��ZnO���������Գ�����ն������Ӷ��������Ч�ʣ��ʵ���ߵ�λʱ����������ѭ������������ʹ�������������գ��Ӷ�����˶������������Ч�ʣ��������ͼ���֪������Һ��pH��6.8����Ҳ���SO2������Ч�ʣ�
��ab����Һ��pH����4��6֮�䣬���ͼ2��֪����pH��������Һ����Ҫ����������������ӣ���ͼ1�е�pH-t����ab�η�������Ҫ��ѧ����ʽΪZnSO3+SO2+H2O=Zn(HSO3)2��
��pH=7ʱ������ͼ2��֪��Һ����������������������Ũ����ȣ�����ݵ���غ�2c(Zn2+)+c(H+)=c(OH-)+c(HSO3-)+2c(SO32-)��֪��Һ��![]() ��
��
��3��������SO2�õ�����ת��ΪS���ʣ���缫��ӦʽΪSO2+4H++4e-=S��+2H2O��
��������SO2ʧȥ����ת��Ϊ���ᣬ�ܷ�ӦʽΪ3SO2+2H2O![]() 2H2SO4+S����19.2g SO2�����ʵ�����0.3mol����������ϻ���S��H2SO4�����ʵ����ֱ�Ϊ0.1mol��0.2mol��
2H2SO4+S����19.2g SO2�����ʵ�����0.3mol����������ϻ���S��H2SO4�����ʵ����ֱ�Ϊ0.1mol��0.2mol��


 ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������(NaBiO3���Ƿ�����ѧ�е���Ҫ�Լ�����������ˮ������ˮ������Ѹ�ٷֽ⡣ij��ѧ��ȤС�������ͼʵ��װ����ȡ�����ơ�װ�ñ���ʢ��Bi(OH)3(������ˮ����NaOH��Һ��������˵���������
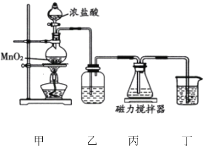
A.װ������ʢװ���Լ��DZ���ʳ��ˮ
B.װ�ñ��з�����Ӧ�����ӷ���ʽ�ǣ�Bi(OH)3+3OH-+Na++Cl2=NaBiO3��2Cl-+3H2O
C.��װ�ñ��л�ò�Ʒ�IJ���Ϊ���ڱ�ˮ����ȴ�ᾧ�����ˡ�ϴ�ӡ�����
D.Ϊ��ȥ���ù����л��е�Bi(OH)3������ϡ����ϴ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӵļ��鷽����ȷ����
A.ij��Һ![]() ������ɫ����,˵��ԭ��Һ����Cl-
������ɫ����,˵��ԭ��Һ����Cl-
B.ij��Һ![]() ������ɫ����,˵��ԭ��Һ����SO42-
������ɫ����,˵��ԭ��Һ����SO42-
C.ij��Һ![]() ������ɫ����,˵��ԭ��Һ����CO32-
������ɫ����,˵��ԭ��Һ����CO32-
D.ij��Һ![]() ������ɫ����,˵��ԭ��Һ����Cu2+
������ɫ����,˵��ԭ��Һ����Cu2+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����V1mL1.0mol/L HCl��Һ��V2mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ��ʵ����ʼ�ձ���V1��V2��50mL����������������ȷ����
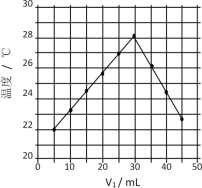
A.����ʵ��ʱ�����¶ȵ���22��
B.��ʵ�������ѧ�ܿ���ת��Ϊ����
C.��ʵ�������ˮ���ɵķ�Ӧһ���Ƿ��ȷ�Ӧ
D.NaOH��Һ��Ũ��ԼΪ1.5mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڻ�ѧ��Ӧ�У�ֻ�м�����������ƽ�������ߵö�ķ�Ӧ����ӷ�����ײʱ�ſ��ܷ�����ѧ��Ӧ����Щ���ӱ���Ϊ����ӡ�ʹ��ͨ���ӱ�ɻ���������ṩ������ȵ������л�ܣ��䵥λͨ����kJ��mol-1��ʾ��������۲���ͼ��Ȼ��ش����⡣
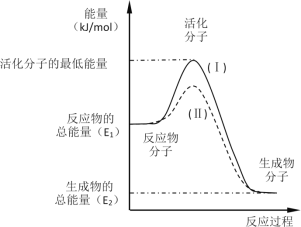
(1)ͼ����ʾ��Ӧ��_________(��������������������)��Ӧ���÷�Ӧ_________(������Ҫ����������Ҫ��)���ȣ��÷�Ӧ����H =___________(�ú�E1��E2�Ĵ���ʽ��ʾ)��
(2)��֪�Ȼ�ѧ����ʽ��H2(g)+![]() O2(g)=H2O(g)����H =-241.8 kJ��mol-1���÷�Ӧ�Ļ��Ϊ167.2 kJ��mol-1�������淴Ӧ�Ļ��Ϊ____________________��
O2(g)=H2O(g)����H =-241.8 kJ��mol-1���÷�Ӧ�Ļ��Ϊ167.2 kJ��mol-1�������淴Ӧ�Ļ��Ϊ____________________��
(3)��֪4��H2ȼ������Һ̬ˮʱ����Ϊ571.6kJ����д����ʾH2ȼ���ȵ��Ȼ�ѧ����ʽΪ��_________
(4)̼ȼ�յ��Ȼ�ѧ����ʽΪ�� C(s)+O2(g)=CO2(g)����H= -393.5kJ/mol��ͨ������˵����������������̼ȼ��ʱ���������ı���_____________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ag����������������ɵĻ�����ܽ���������ϡ�����У�����õ�bg��������ԭ�����������������������Ϊ�� ��
A.5bgB.(a5b) gC.(a 11b/4)gD.11b/4g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1.4�˴����۷���80����1mol/L��ϡ�����У���Ӧֹͣ�����ɵ���������ʲô��___�ж��ٿˣ�____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��A��H��Ϊ��ѧ��ѧ�г��������ʣ�A��B��HΪ���壬��Ӧ������Ҫ�Ĺ�ҵ��Ӧ������֮��������ת����ϵ(��Ӧ�����ɵ�ˮ����ȥ)��

��ش��������⣺
��1��B��_____��D��_____��G��_____��H��_____(�ѧʽ)��
��2����ҵ�ϳ����÷�Ӧ����ȡƯ�ۣ��÷�Ӧ�Ļ�ѧ����ʽ��__________��Ư������ˮ���ܿ����е�CO2���ã���������Ư�ס�ɱ�����õĴ����ᣬ��ѧ��Ӧ����ʽΪ_________��
��3��A��Ԫ�ص�ԭ�ӽṹʾ��ͼΪ________��
��4��F����Һ�еĵ��뷽��ʽΪ_________��
��5��������Ӧ������������ԭ��Ӧ����_______(��д���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˱���NO��NO2��N2O4�Դ�������Ⱦ��������NaOH��Һ�������մ���(��Ӧ����ʽ��2NO2��2NaOH===NaNO3��NaNO2��H2O��NO2��NO��2NaOH===2NaNO2��H2O)��������a mol NO��b mol NO2��c mol N2O4��ɵĻ������ǡ�ñ�V L NaOH��Һ����(������ʣ��)�����NaOH��Һ�����ʵ���Ũ��Ϊ(����)
A. ![]() mol��L��1 B.
mol��L��1 B. ![]() mol��L��1
mol��L��1
C. ![]() mol��L��1 D.
mol��L��1 D. ![]() mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com